Polymethylene-bridged ferrocenyl bipyrazole and synthetic method thereof
A technology of methylene bridged ferrocenyl bispyrazoles and synthetic methods, which is applied in the fields of chemical instruments and methods, metallocenes, organic chemistry, etc., and can solve the problem of inability to synthesize ferrocenyl bispyrazoles and the efficiency of synthetic methods No high-level problems, to achieve the effect of excellent target product yield, high target product yield, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
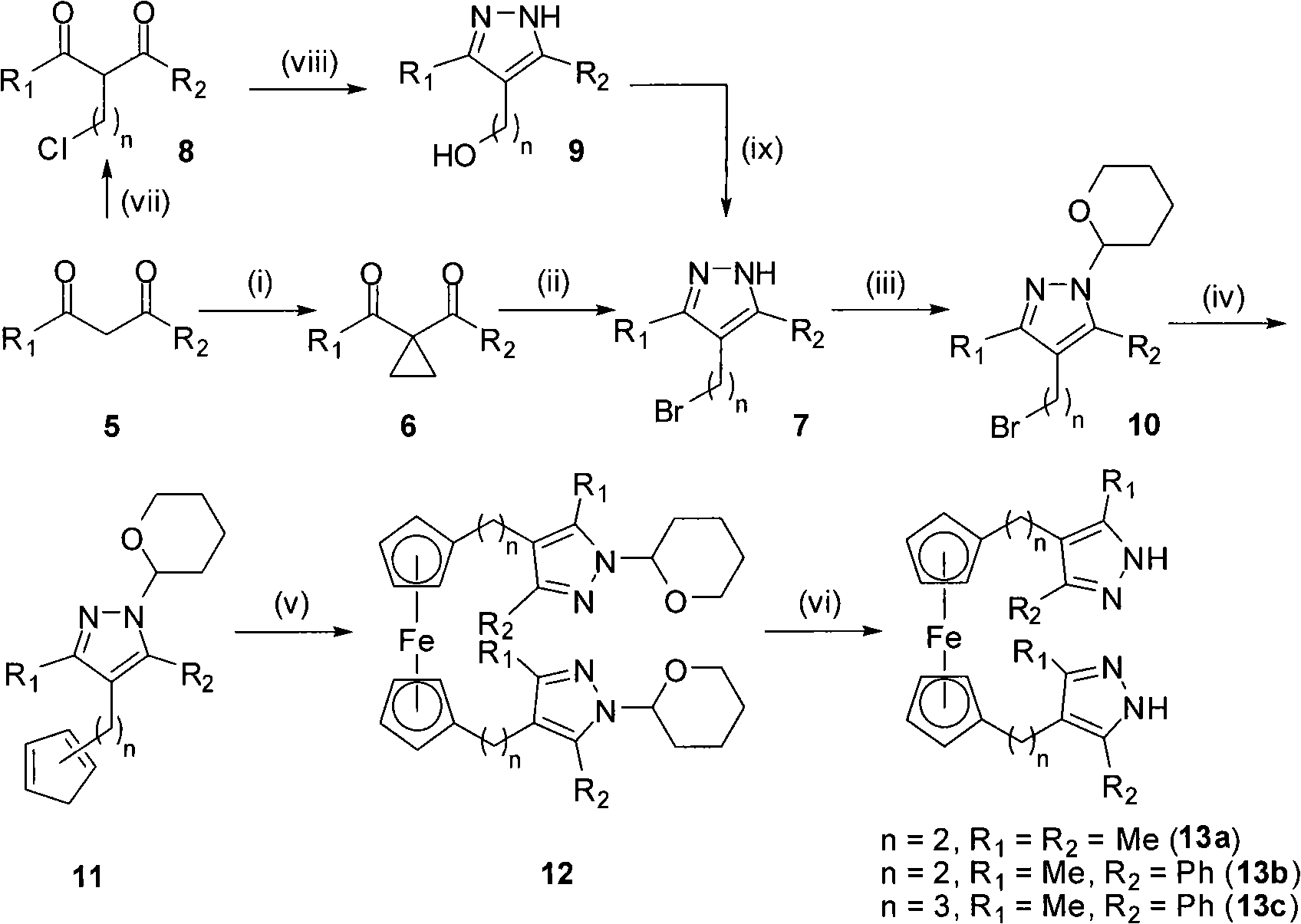
Image
Examples
Embodiment 1
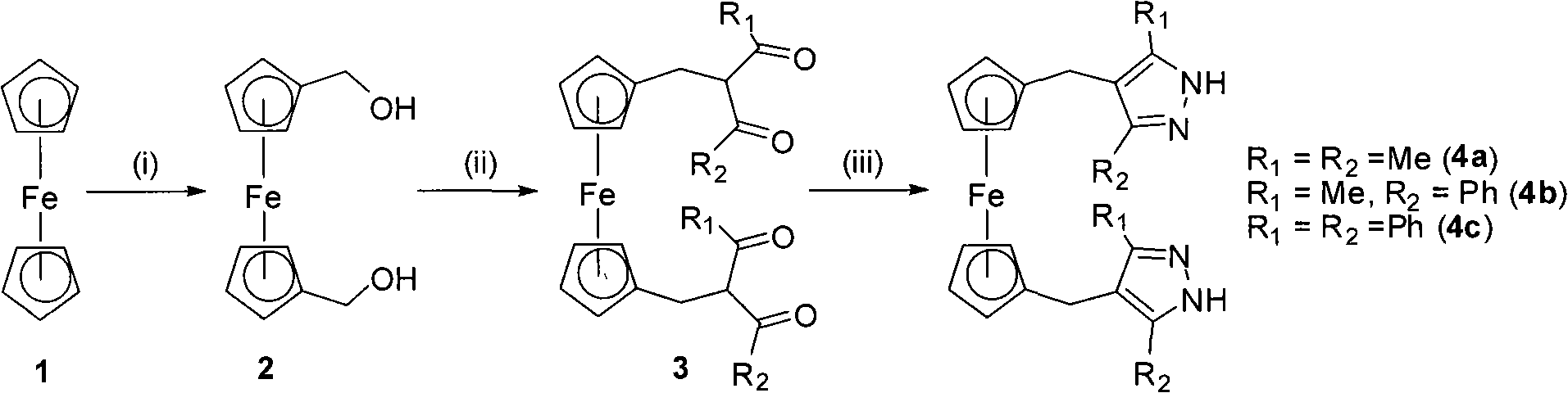
[0034] Example 1: Synthesis of 1,1'-ferrocenedimethanol 2
[0035] In a 250mL branched flask, N 2 Replaced three times, dissolved ferrocene (5.0g, 26.9mmol) in 80mL of anhydrous ether, treated with n-butyl lithium (2.5 Minhexane, 22.4mL, 56.0mmol) at room temperature, reacted at room temperature for 2h, then added TMEDA dropwise (3.2g, 27.5mmol), stirred at room temperature for 24h. Paraformaldehyde (2.0 g, 66.6 mmol) was added in batches, stirred at room temperature for 24 h, cooled to 0 °C, quenched with 50 mL of saturated aqueous ammonium chloride, extracted with 200 mL of dichloromethane, and dried with anhydrous magnesium sulfate. Filtration, concentration under reduced pressure, separation and purification by column chromatography, the chromatographic column is a silica gel column, the eluent is petroleum jelly (60-90°C) / ethyl acetate (v / v, 1:1), and the orange-yellow component is collected 2. The target product yield is 41%.
Embodiment 2
[0036] Example 2: Synthesis of 1,1'-bis(1-phenyl-3-methyl-1,3-butanedione)ferrocene 3b
[0037] In a 25mL branched flask, N 2 Replaced three times, added ferrocenyl diol compound 2 (1.00g, 4.06mmol) and benzoylacetone (1.32g, 8.12mmol), then added 2.0mL dichloromethane to dissolve it, and dissolved 1.0mL 40% hydrofluoric Acid (28.5mmol) was added dropwise, reacted at room temperature for 30min, and then diluted with 100mL ether. The organic phase was washed with 5×50 mL of water, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure, and dried in vacuo to obtain orange-yellow oil 3b with a yield of 79%.
Embodiment 3
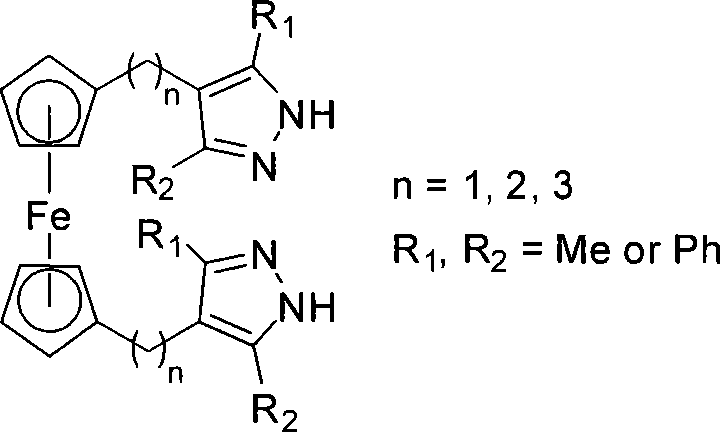
[0038] Example 3: Synthesis of 1,1'-bis(3-methyl-4-methyl-5-phenylpyrazole)ferrocene 4b
[0039] In a 50mL branched flask, N 2 Replaced three times, dissolved ferrocenylbis(1,3-diketone) 3b (1.50g, 2.70mmol) in 40mL of absolute ethanol, added 85% hydrazine hydrate (2.0mL, 34.0mmol), stirred at reflux for 3h, Cool to room temperature, concentrate under reduced pressure, and then separate and purify by column chromatography. The chromatographic column is a silica gel column, and the eluent is petroleum jelly (60-90 ℃) / ethyl acetate (v / v, 1:1). Component 4b, the yield is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com