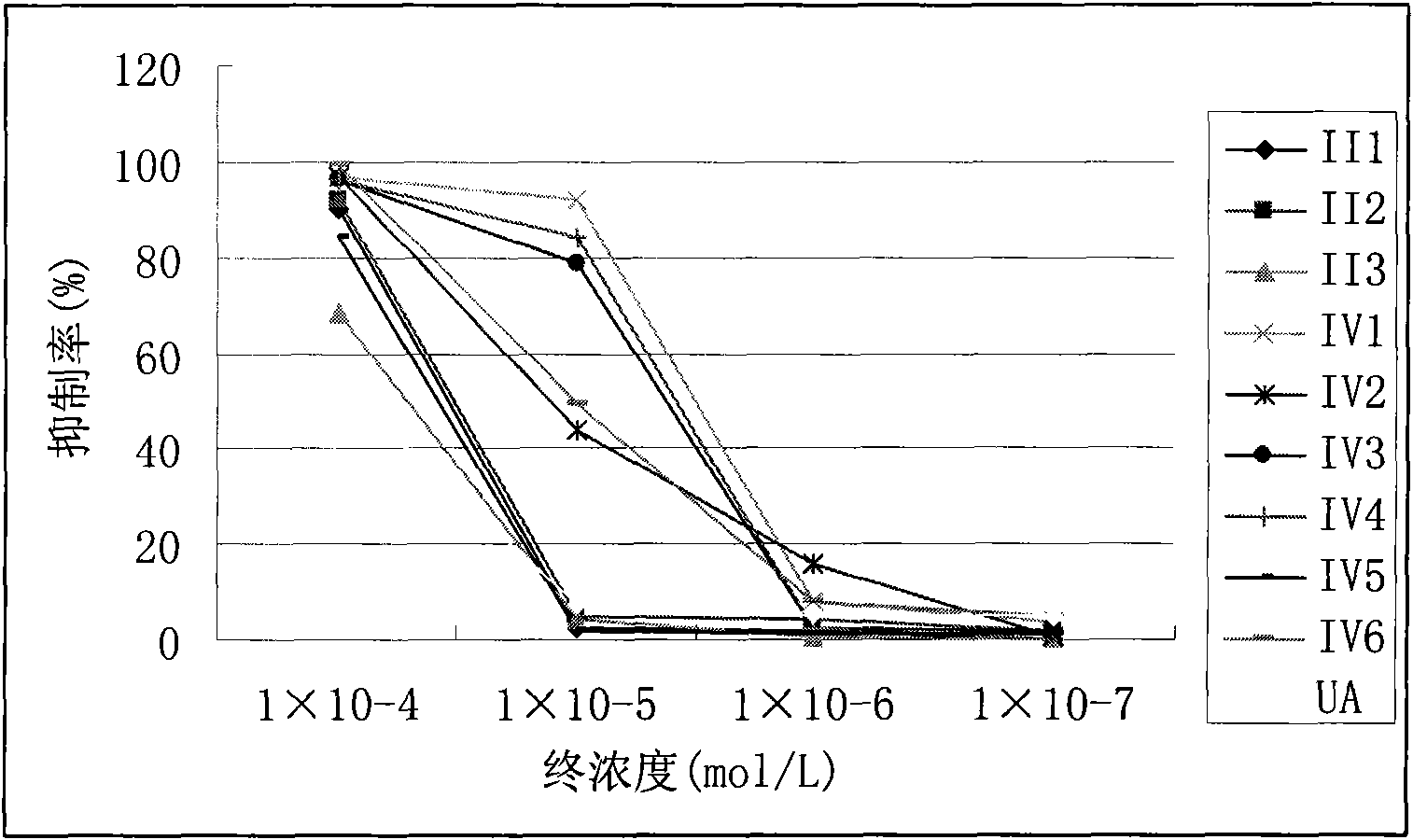
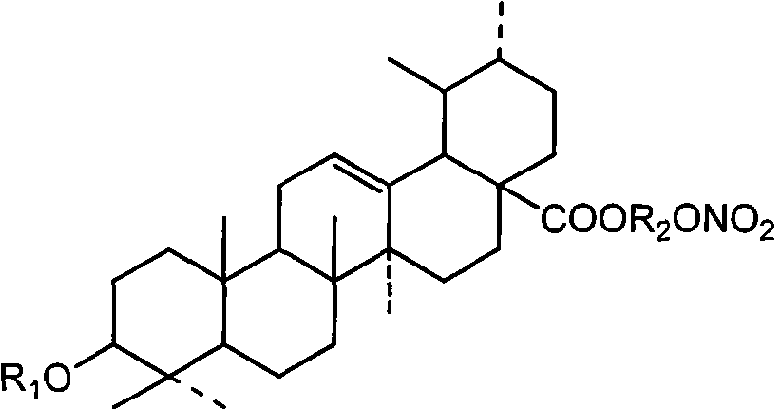
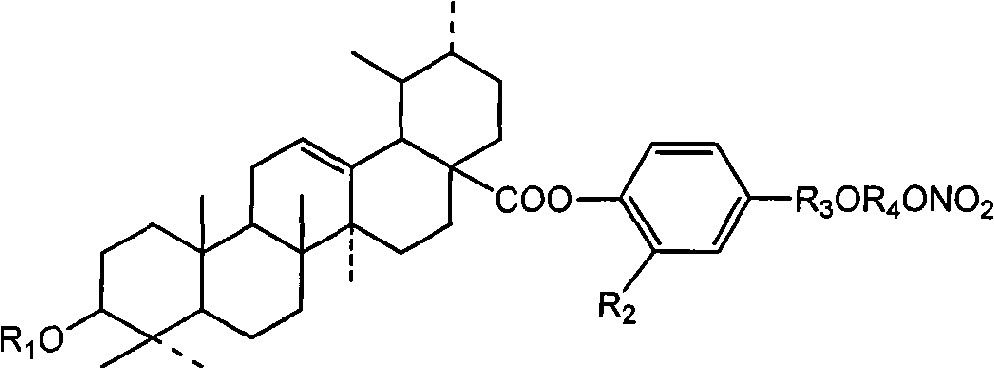
Synthesizing method of Ursolic Acid (UA) derivative and application thereof in pharmacy
A compound and representative technology, applied in the application field of preparing drugs for inducing liver tumor cell apoptosis, can solve the problems of affecting clinical efficacy, low bioavailability, poor solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Ursolic acid-3-nitrooxypropyl ester (I 2 )
[0048] UA (500mg, 1.05mmol) was suspended in 15ml of acetone, and triethylamine (2ml) was added to dissolve it completely, and 1,3-dibromopropane (0.5ml, 6mmol) was added under stirring at room temperature, and stirred and refluxed for 12 hours to obtain intermediate Ursolic acid-3-bromopropyl ester, then reflux with silver nitrate in the dark for 6h to filter out insoluble matter, concentrate the filtrate, and perform silica gel column chromatography [ethyl acetate:petroleum ether (60~90°C)=1:4(V : V)], 150mg of white solid was obtained, yield 65%, m.p.76~78℃.
[0049] ESI-MS: 568[M+Na] +
[0050] IR (KBr, cm -1 ): 3474 (OH), 1720 (C=O), 1637 (ONO 2 )
[0051] 1 H NMR (300MHz, CDCl 3 ), δ: 0.71 (s, 3H, CH 3 ), 0.78(s, 3H, CH 3 ), 0.88(s, 6H, 2×CH 3 ), 0.90 (d, 3H, CH 3 ), 0.97 (d, 3H, CH 3 ), 1.00 (s, 3H, CH 3 ), 3.20(m, 1H, 3α-H), 4.13(t, 2H, OCH 2 , J=6Hz), 4.54(t, 2H, OCH 2 , J=6Hz), 5.25~5.27(m, 1H, C 12...
Embodiment 2
[0056] (1) 4-hydroxyl-3-methoxyphenyl acrylate-2-bromoethyl ester (2a 1 )
[0057] Ferulic acid (5g, 25.8mmol) was dissolved in 50ml of acetone, 1,2-dibromoethane (22.26g, 100mmol) and 10ml of triethylamine were added, heated at an external temperature of 50°C for 4 hours, cooled to room temperature, A large amount of white solid was precipitated, filtered, the filtrate was concentrated, column chromatography [ethyl acetate:petroleum ether (60-90°C)=1:4 (V:V)], 7.8g of needle-like solid was obtained, yield 67% , m.p.102-104°C.
[0058] In the same way, other intermediates such as 2a can be prepared from ferulic acid, vanillic acid, p-hydroxycinnamic acid and other dibromoalkanes, dibromoalkenes, and dibromoalkynes.
[0059] (2) 3-O-trifluoroacetyl-ursolic acid-2-methoxy-4-[2-(2-bromo-ethoxycarbonyl)-vinyl]-phenyl ester (2b 1 )
[0060] UA (456mg, 1mmol) was suspended in toluene, trifluoroacetic anhydride (1ml, 7.42mmol) was added under stirring, the reaction solution was c...
Embodiment 3
[0069] (1) 4-hydroxyl-3-methoxyphenyl acrylate-2-bromopropyl ester (2a 2 )
[0070] Refer to 2a 1 The preparation method is prepared from ferulic acid and 1,3-dibromopropane, with a yield of 68%, m.p.100-102°C.
[0071] (2) 3-O-trifluoroacetyl-ursolic acid-2-methoxy-4-[2-(3-bromo-propoxycarbonyl)-vinyl]-phenyl ester (2b 2 )
[0072] Refer to 2b 1The preparation method of UA and 8b, column chromatography [ethyl acetate: petroleum ether (60 ~ 90 ° C) = 1: 4 (V: V)] to obtain a yellow oil, yield 74%.
[0073] (3) 3-O-trifluoroacetyl-ursolic acid-2-methoxy-4-[2-(3-nitrooxy-propoxycarbonyl)-vinyl]-phenyl ester (II 3 )
[0074] Reference II 1 The preparation method, by 2b 1 Prepared by reacting with AgNO3 in the dark, column chromatography [ethyl acetate:petroleum ether (60-90°C)=1:4 (V:V)] gave a white solid with a yield of 71.8%, m.p.100-102°C.
[0075] ESI-MS: 849[M+NH 4 ] +
[0076] IR (KBr, cm -1 ): 1778 (C=O), 1754 (C=O), 1720 (C=O), 1634 (ONO 2 )
[0077] 1 H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com