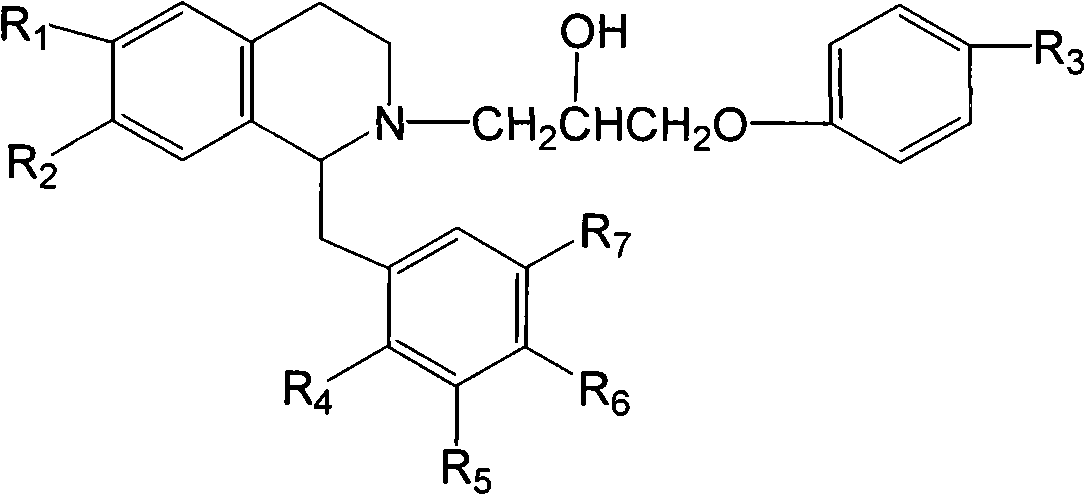
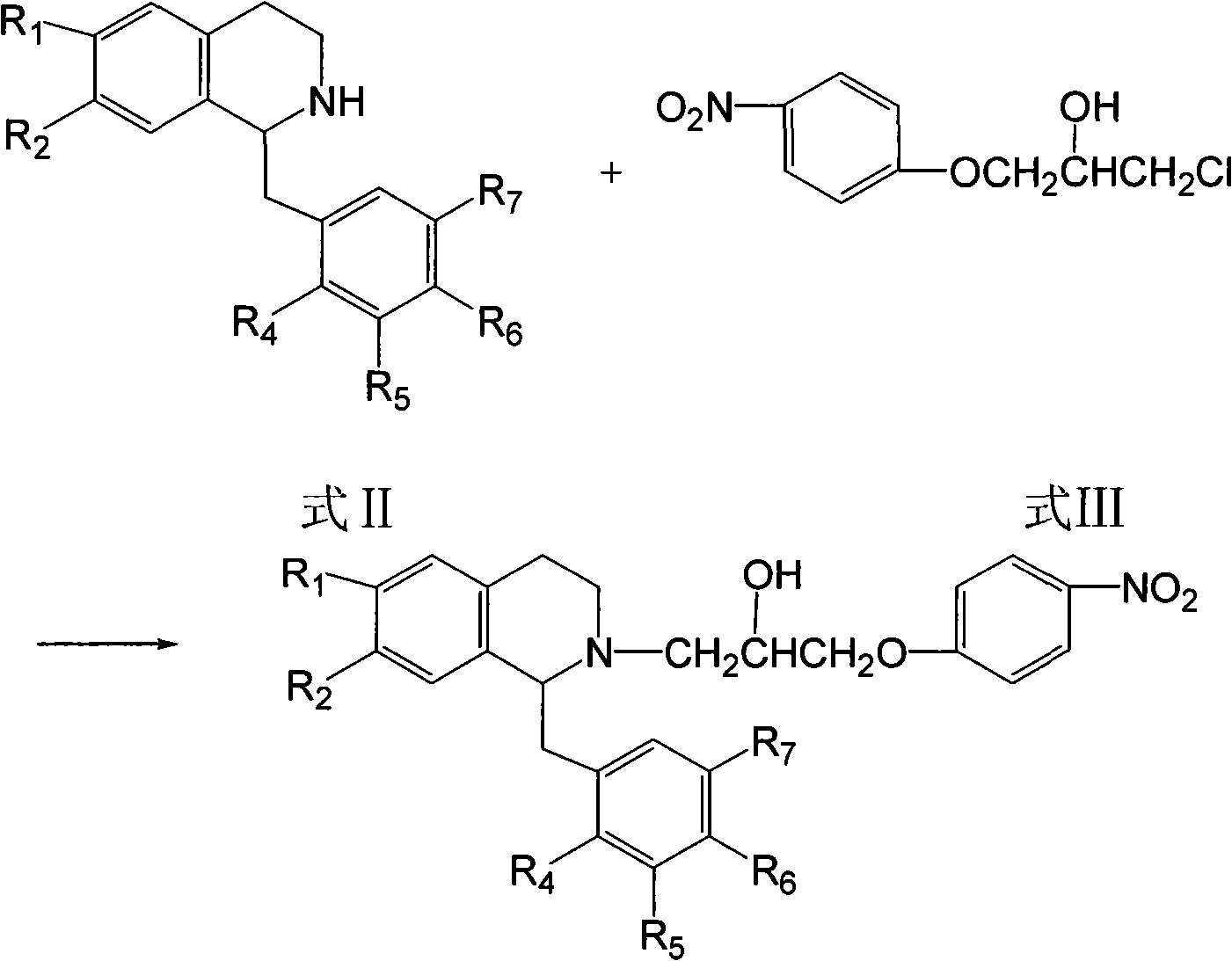
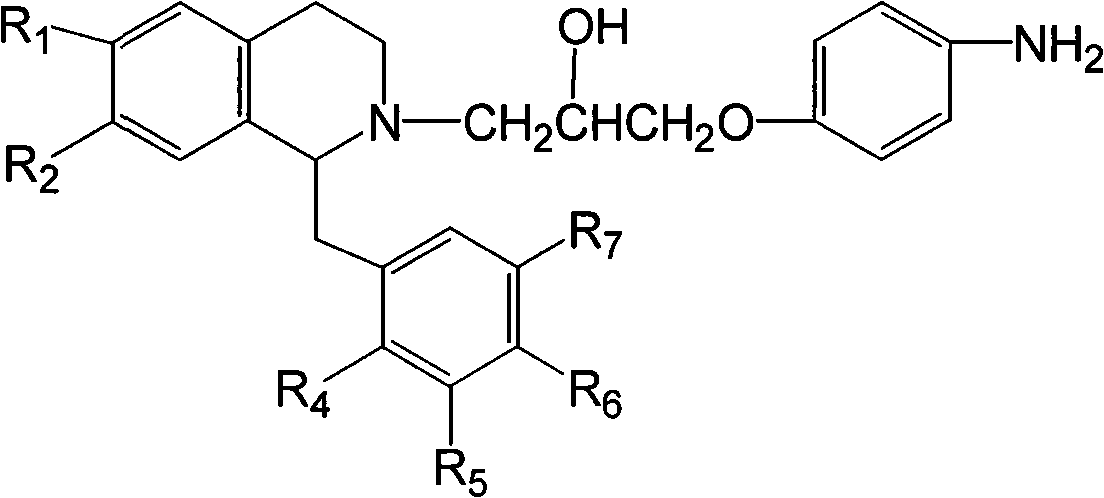
Isoquinoline compound or salt thereof, medicinal composition, preparation method and application thereof
A technology of tetrahydroisoquinoline hydrochloride and compound, applied in the field of isoquinoline compound or its salt, can solve the problems of poor oral absorption, irregularity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 1-p-methoxybenzyl-2-[1-(p-aminophenoxy)-2-hydroxy-propyl]-6,7-dimethoxy-1,2,3,4- Tetrahydroisoquinoline hydrochloride (I-5)
[0060] (1) 1-(p-methoxybenzyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride 0.1mol 3,4-dimethoxybenzene Mix ethylamine with 0.1mol p-methoxyphenylacetic acid, heat at 170-180°C for 4 hours, cool, dissolve in chloroform, wash with 2M HCl, 2M NaOH and water successively, dry over anhydrous magnesium sulfate, evaporate under reduced pressure solvent, the remainder was treated with EtOH-H 2 O was recrystallized to obtain N-(3,4-dimethoxyphenethyl)-4-oxyphenylacetamide.
[0061] Dissolve 0.05 mol of the above amide in dry chloroform, add 26 ml of phosphorus oxychloride, reflux for 4 hours, evaporate the solvent and excess phosphorus oxychloride under reduced pressure, wash the residue with petroleum ether to obtain a solid, and recrystallize from ethanol to obtain 1-(p-methoxybenzyl)-6.7-dimethoxy-3,4-dihydroisoquinoline hyd...
Embodiment 2
[0067] Example 2 1-p-methoxybenzyl-2-[1-(p-aminophenoxy)-2-hydroxy-propyl]-6,7-dimethoxy-1,2,3,4- tetrahydroisoquinoline tartrate salt
[0068] The 1-p-methoxybenzyl-2-[1-(p-aminophenoxy)-2-hydroxy-propyl]-6,7-dimethoxy-1,2 prepared in Example 1, Dissolve 3,4-tetrahydroisoquinoline in ethyl acetate, add saturated 8wt% tartaric acid ethyl acetate solution dropwise to about pH=1.5, stir to precipitate crystals, filter, wash the filter cake with ethyl acetate, and dry , to get 1-p-methoxybenzyl-2-[1-(p-aminophenoxy)-2-hydroxy-propyl]-6,7-dimethoxy-1,2,3,4-tetra Hydroisoquinoline tartrate.
Embodiment 3
[0069] Example 3 1-p-methoxybenzyl-2-[1-(p-aminophenoxy)-2-hydroxy-propyl]-6,7-methylenedioxy-1,2,3,4 -Tetrahydroisoquinoline hydrochloride (I-11)
[0070] (1) 1-(p-methoxybenzyl)-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline hydrochloride
[0071] According to the method of step (1) in Example 1, 3,4-dimethoxyphenethylamine is replaced by piperonyl ethylamine to obtain 1-(p-methoxybenzyl)-6,7-methylenedi Oxy-1,2,3,4-tetrahydroisoquinoline hydrochloride. The melting point is 113-116°C.
[0072] (2) 1-(p-methoxybenzyl)-2-[1-(p-nitrophenoxy)-2-hydroxy-propyl]-6,7-methylenedioxy-1,2, 3,4-tetrahydroisoquinoline hydrochloride (I-10)
[0073] 3.65g (8.57mmol) of 1-(p-methoxybenzyl)-6,7-methylenedioxy-1,2,3,4-tetrahydroisoquinoline hydrochloride, compound (Formula III) 4g (17.14mmol), KOH1.63g (29.14mmol), 100ml volume ratio 91% methanol and NaI 0.26g (1.71mmol) mixed, heated to reflux, thin-layer chromatography detected that the reactant was consumed and stopped the reactio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com