Application of N-hydroxy compound containing nitrogen aromatic ring in hydrocarbon oxidation
An aromatic ring nitrogen hydroxyl compound technology, applied in the field of hydrocarbon oxidation reaction, can solve the problems of few types of organic nitrogen hydroxyl compounds, prone to excessive oxidation reaction, cumbersome, requiring four to five steps, etc., and achieves easy operation and high goals. Product selectivity, effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
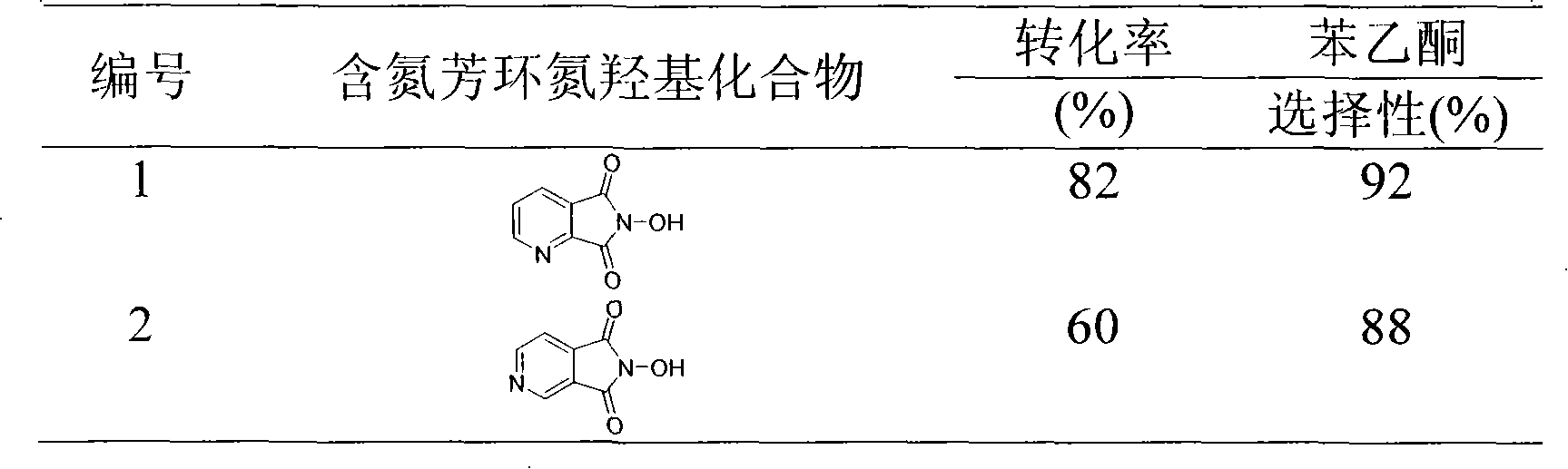
[0020] Example 1: Synthesis of 6-hydroxypyrrolo[3,4-b]pyridine-5,7-dione
[0021] Add anhydrous sodium carbonate and hydroxylamine hydrochloride to the flask, add glacial acetic acid, and heat to reflux in an oil bath for 5 minutes. Add pyridine dicarboxylic anhydride and heat to reflux. Cool slightly after the reaction, pour into a small beaker, let it stand for cooling, crystals are precipitated, and the target product is obtained by suction filtration with a yield of 50%. It was identified as 6-hydroxypyrrolo[3,4-b]pyridine-5,7-dione by IR and NMR. 1 H NMR (400MHz, DMSO-d 6 )δ11.01(s, 1H), 8.95(dd, 1H, J=5.2, 1.6Hz), 8.25(dd, 1H, J=7.6, 1.2Hz), 7.77(dd, 1H, J=7.6, 5.2Hz ); 13 C NMR: (400MHz, DMSO-d 6 )δ162.6, 154.6, 149.0, 130.9, 128.0, 125.3; IR (KBr, neat) 3082, 1747, 1724, 1607, 1566, 1387, 1155, 1101cm -1 .
Embodiment 2
[0022] Example 2: Synthesis of 6-hydroxypyrrolo[3,4-b]pyrazine-5,7-dione
[0023] Add anhydrous potassium carbonate and hydroxylamine hydrochloride into a two-necked flask, add acetic anhydride, and heat to reflux for 5 minutes. Add pyridine dicarboxylic anhydride and heat to reflux. After the reaction was finished and cooled slightly, it was poured into a small beaker, left to cool, crystals were precipitated, and the yield was 30%. It was identified as 6-hydroxypyrrolo[3,4-b]pyrazine-5,7-dione by IR and NMR. 1 H NMR (400MHz, DMSO-d 6 )δ11.31(s, 1H), 8.96(s, 1H); 13 C NMR: (400MHz, DMSO-d 6 )δ161.2, 148.3, 145.4; IR (KBr, neat) 3087, 1751, 1731, 1608, 1535, 1369, 1274, 1164, 1118cm -1 .
Embodiment 3
[0024] Embodiment 3: air oxidation of tetralin
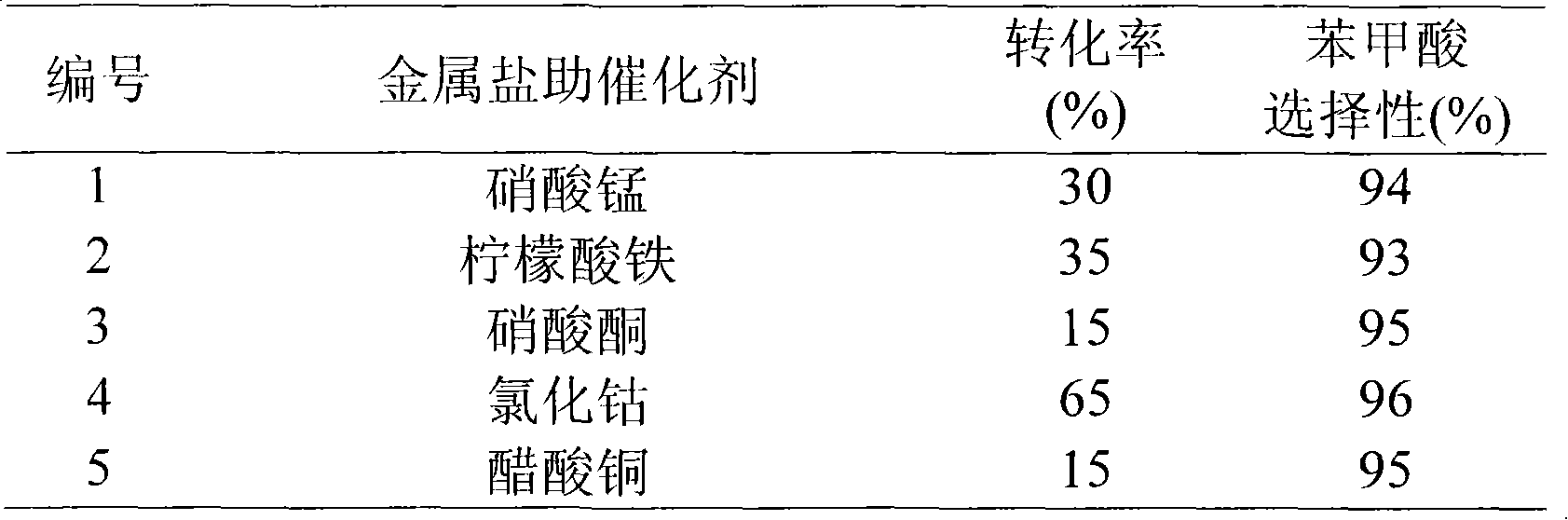
[0025] In a 100 ml three-necked flask, put 20 ml of tetralin and 0.3 g of 6-hydroxypyrrolo[3,4-b]pyridine-5,7-dione; heat up to 80°C while stirring, and insert the reaction solution The air guide tube inside is fed into air continuously, and the air flow rate is 50 ml / min, and the excess air is discharged from the serpentine condensing tube, reacted for 15 hours, and analyzed the product composition with a gas chromatograph. As a result, the conversion of tetralin was 80%, and the selectivity of α-tetralone was 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com