Preparation method of glycerin and fructose injection
A technology for injection and fructose, applied in the field of medical injection, can solve the problems of yellowing of solution color, accelerated fructose degradation, damage, etc., and achieve the effect of shortening heating time and reducing degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
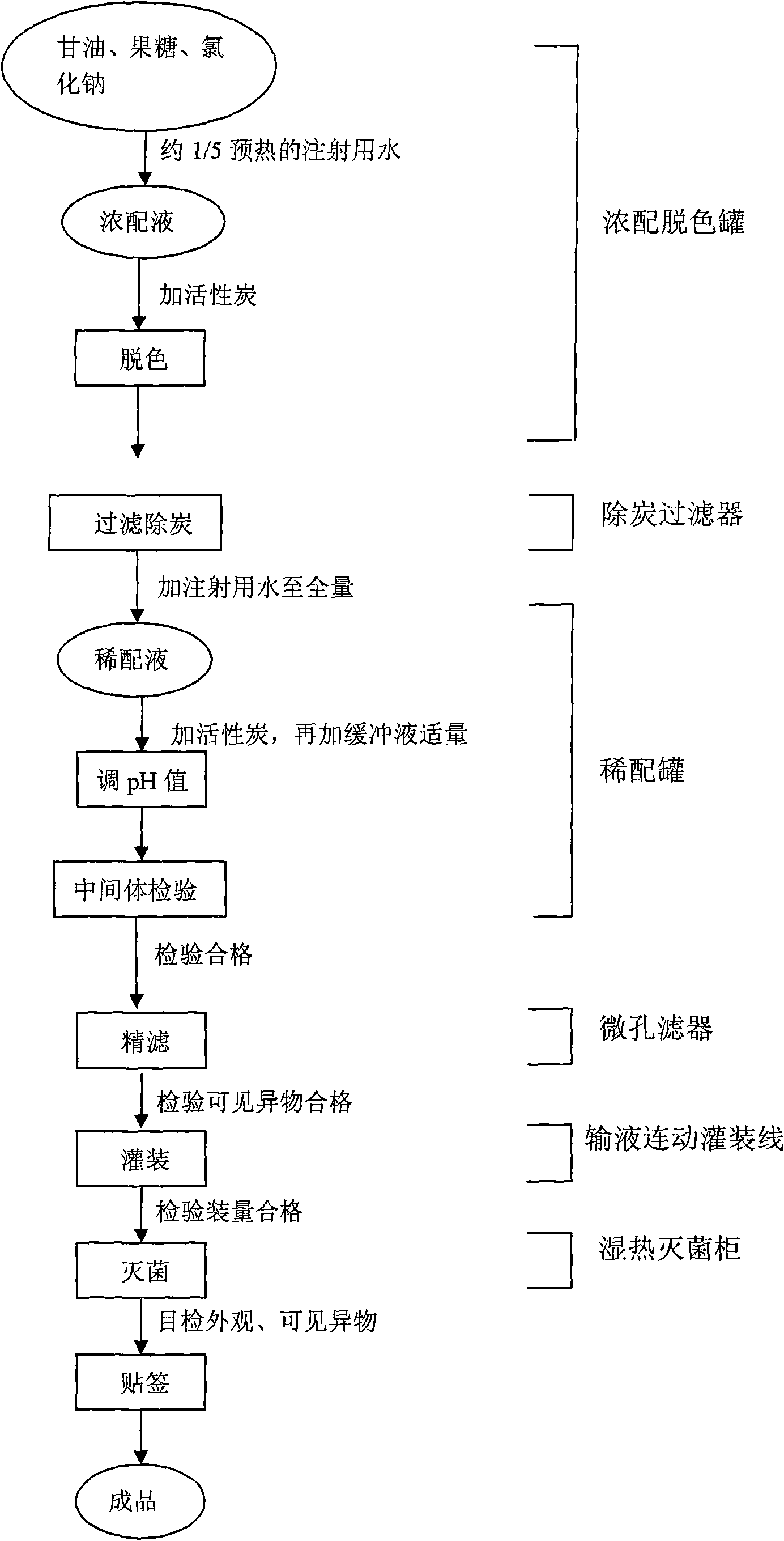
[0025] a) Dissolve 100g of glycerin, 50g of fructose and 9g of sodium chloride in about 170ml of water for injection and fully dissolve.
[0026] b) add activated carbon (weight ratio) accounting for about 2 / 10,000 of the total production amount, boil and stir for 15 minutes, and filter to remove carbon.
[0027] c) Add water for injection to the full amount (1000ml), then add activated carbon accounting for 1 / 10,000 of the total amount produced, stir, and adjust the pH value to 5.2 with acetic acid and sodium acetate (1:1) buffer solution. The full production volume mentioned here refers to the total production volume of a batch.
[0028] d) Intermediate inspection (content: 0.873% to 0.927% of sodium chloride, pH value: 4.7 to 5.2). e) Filtration to remove carbon, fine filtration with a 0.45μm filter, check for foreign matter, and fill after passing the test.
[0029] f) Sterilize with moist heat at 121°C for 10 minutes, and cool quickly.
[0030] Among them, the intermed...
Embodiment 2
[0032] a) Dissolve 100g of glycerin, 50g of fructose and 9g of sodium chloride in about 165ml of water for injection and fully dissolve.
[0033] b) Add activated carbon accounting for about 2 / 10,000 of the total production amount, boil and stir for 15 minutes, and filter to remove carbon.
[0034] c) Add water for injection to the full amount (1000ml), then add activated carbon accounting for about 1 / 10,000 of the total amount produced, stir, and adjust the pH value between 5.2 with lactic acid and sodium lactate (1:1) buffer.
[0035] d) Intermediate inspection (content: 0.873% to 0.927% of sodium chloride, pH value: 4.7 to 5.2).
[0036] e) Filtration to remove carbon, fine filtration with a 0.45μm filter, check for foreign matter, and fill after passing the test.
[0037] f) Sterilize with moist heat at 121°C for 10 minutes, and cool quickly.
[0038] Wherein the intermediate inspection method is with embodiment 1.
Embodiment 3
[0040] a) Dissolve 100g of glycerin, 50g of fructose and 9g of sodium chloride in about 170ml of water for injection and fully dissolve.
[0041] b) Add about 2 / 10,000 activated carbon, boil and stir for 15 minutes, filter to remove charcoal.
[0042] c) Add water for injection to the full amount (1000ml), then add about 1 / 10,000th of the total amount of production of activated carbon, stir, and adjust the pH value between 5.2 with acetic acid and sodium acetate (1:1) buffer solution.
[0043] d) Intermediate inspection (content: 0.873% to 0.927% of sodium chloride, pH value: 4.7 to 5.2).
[0044] e) Filtration to remove carbon, fine filtration with a 0.45μm filter, check for foreign matter, and fill after passing the test.
[0045] f) Sterilize with moist heat at 115°C for 30 minutes, and cool quickly.
[0046] Wherein the intermediate inspection method is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com