Pharmaceutical composition containing micronized human vascular endostatin
A technology of vascular endothelium and composition, which is applied in the field of preparation of human vascular endostatin pharmaceutical composition, can solve the problems of low solubility of protein and polypeptide drugs, achieve the effects of reducing the number of administrations, facilitating adjustment, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
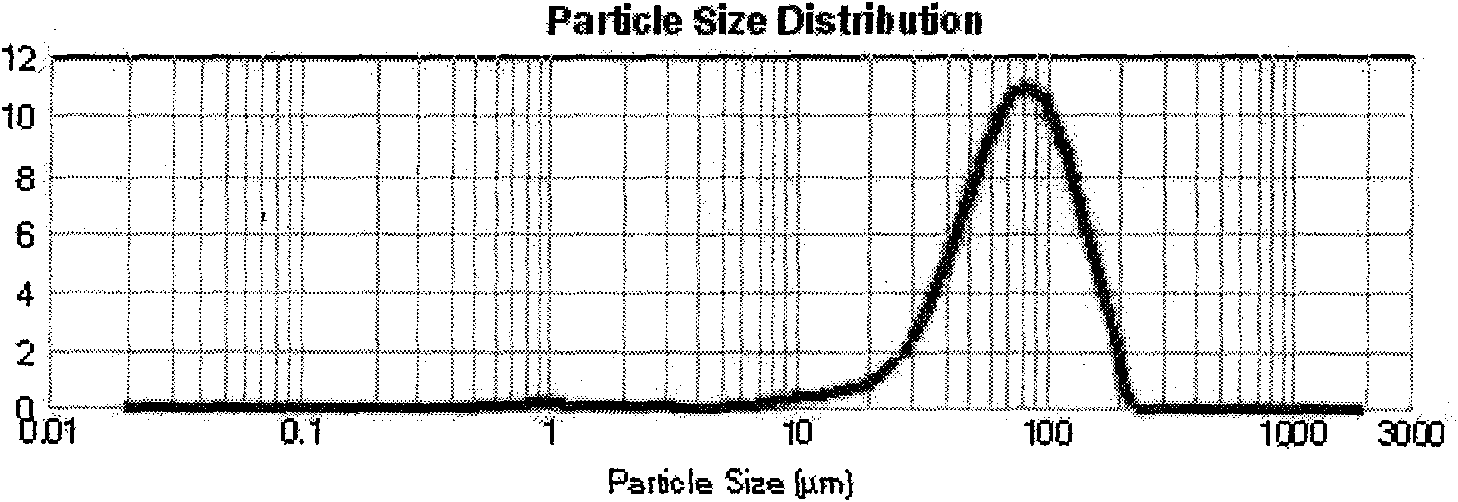
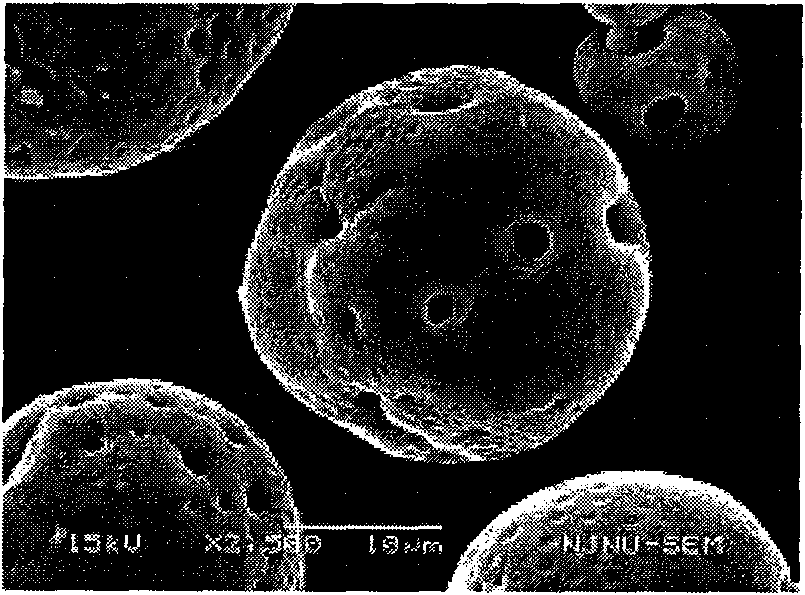
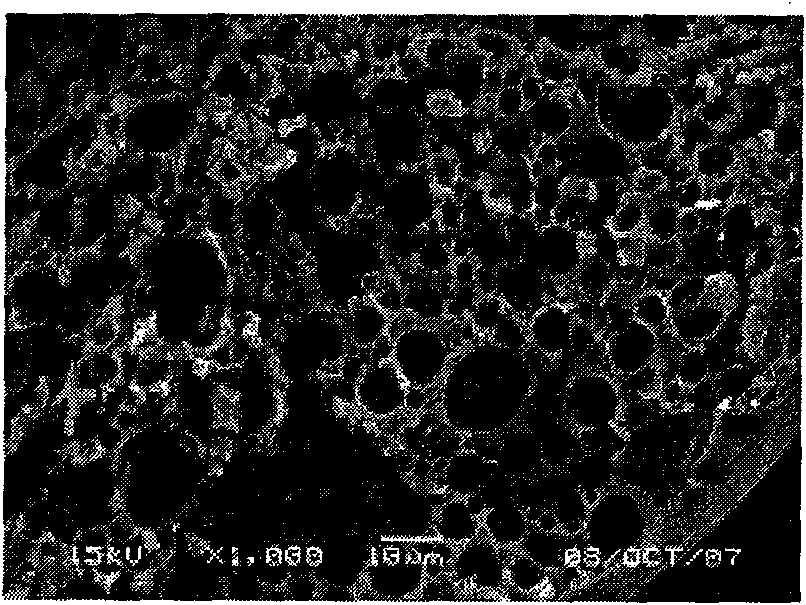
[0041] Precisely measure 50ml of recombinant human endostatin Endostar protein solution (9.1462mg / ml, PH=5.5), add 0.004% zinc acetate, until the zinc salt is completely dissolved, regulate the protein with sodium hydroxide solution (1mol / L) The pH of the solution is in the range of 8.8-9.3. At this point, a large amount of protein precipitated, and the solution was milky white. Add 50ml of PEG-8000 solution with a concentration of 50% (w / v), and stir magnetically for 5 minutes to make it evenly mixed. The above-prepared solution was divided into 30ml vials, the filling specification was 5ml / bottle, and freeze-dried. Dissolve the freeze-dried product with dichloromethane, dissolve PEG-8000 in dichloromethane, and after centrifugation, obtain micronized protein containing dichloromethane at the bottom of the centrifuge tube, dry it under reduced pressure at room temperature, and obtain dry micronized protein. 2 g of PLGA (Mw=50000, lactic acid:glycolic acid=50:50) was dissolve...
Embodiment 2
[0045] Accurately measure 50ml of recombinant human endostatin protein Endostar solution (9.1462mg / ml, PH=5.5), add 0.004% zinc acetate, until the zinc salt is completely dissolved, regulate the protein with sodium hydroxide solution (1mol / L) The pH of the solution is in the range of 8.8-9.3. At this point, a large amount of protein precipitated, and the solution was milky white. Add 50ml of PEG-8000 solution with a concentration of 50% (w / v), and stir magnetically for 5 minutes to make it evenly mixed. The above-prepared solution was divided into 30ml vials, the filling specification was 5ml / bottle, and freeze-dried. Dissolve the lyophilized product with dichloromethane, and dissolve PEG-8000 in dichloromethane. After centrifugation, micronized protein containing dichloromethane is obtained at the bottom of the centrifuge tube, and dried under reduced pressure at room temperature to obtain dry micronized protein. The composition powder was dissolved with 2 ml of dichlorometh...
Embodiment 3
[0049] Get the microsphere prepared by embodiment two to carry out the biological activity assay of Endostar
[0050] 1. HUVEC cells were cultured with ECM supplemented with FBS, ECGS, and P / O Solution at 37°C, 5% CO 2 Cultured in an incubator, and inoculated after the cells were in good condition and entered the logarithmic growth phase.
[0051] 2. Digest the cells with 0.25% trypsin, centrifuge at 1000rpm for 5min, discard the supernatant, resuspend with the medium, and count live cells with a hemocytometer under a microscope. Adjust the cell density to 5000 cells / ml, add 160 μl of cell suspension to each well, and store at 37°C in 5% CO 2 cultured in an incubator.
[0052] 3. Pre-dilute the endostar stock solution and the extract obtained during the determination of the content of Endostar microspheres with pH 7.4 phosphate buffer to 2.5mg / ml, and the final concentration in the well is 500, 250, 125, 62.5, 31.25, 15.625 , 0μg / ml, add 40μl drug volume to each well, set 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume average particle size | aaaaa | aaaaa |
| Volume average particle size | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com