Melamine hapten and antigen as well as preparation method and application thereof
A melamine and antibody technology, which is applied to the preparation method of peptides, chemical instruments and methods, animal/human proteins, etc., to achieve the effect of simple sample pretreatment, low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, preparation and identification of melamine hapten and melamine antigen
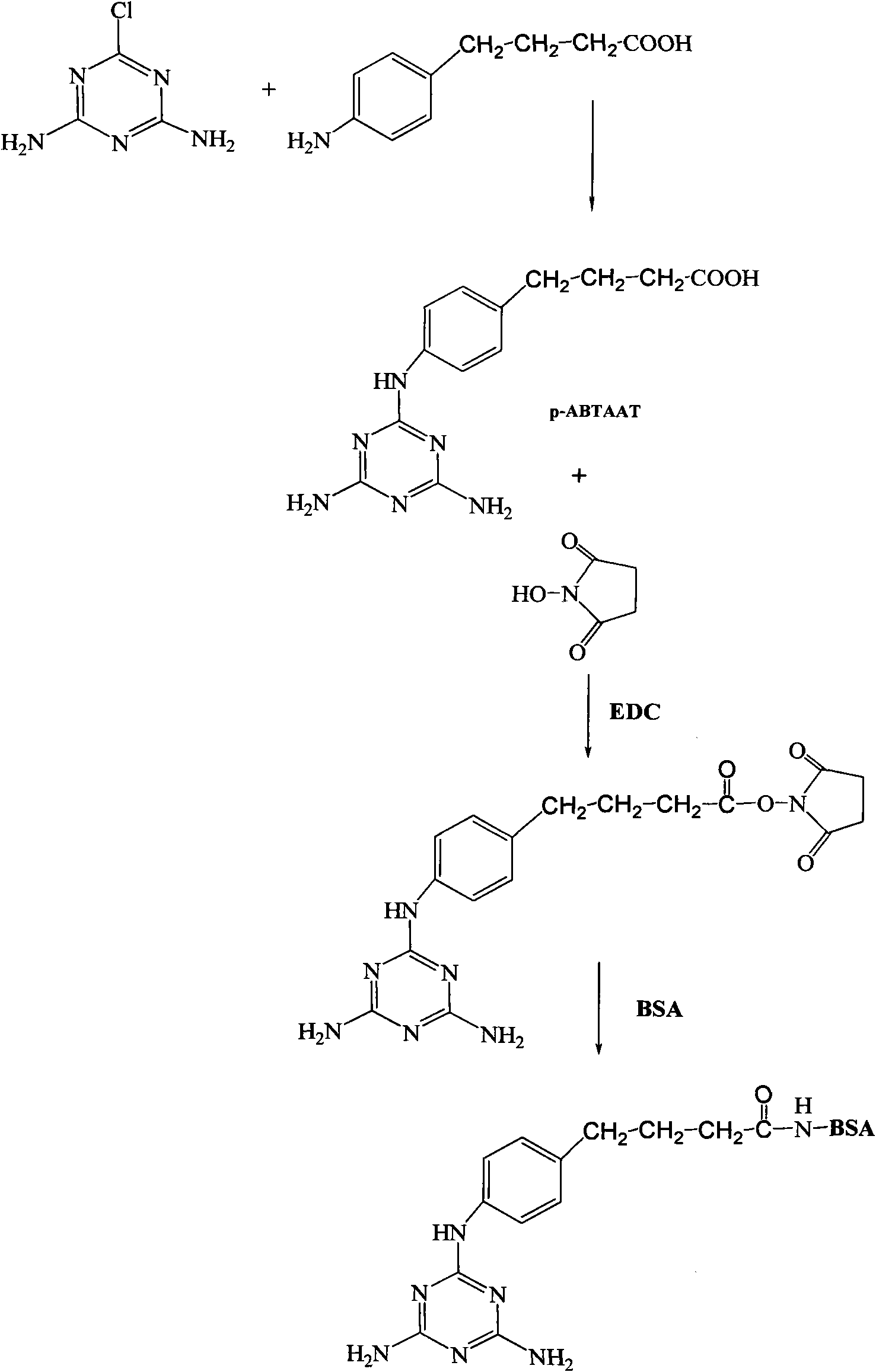
[0032] Synthesis of melamine hapten and antigen figure 1 shown.
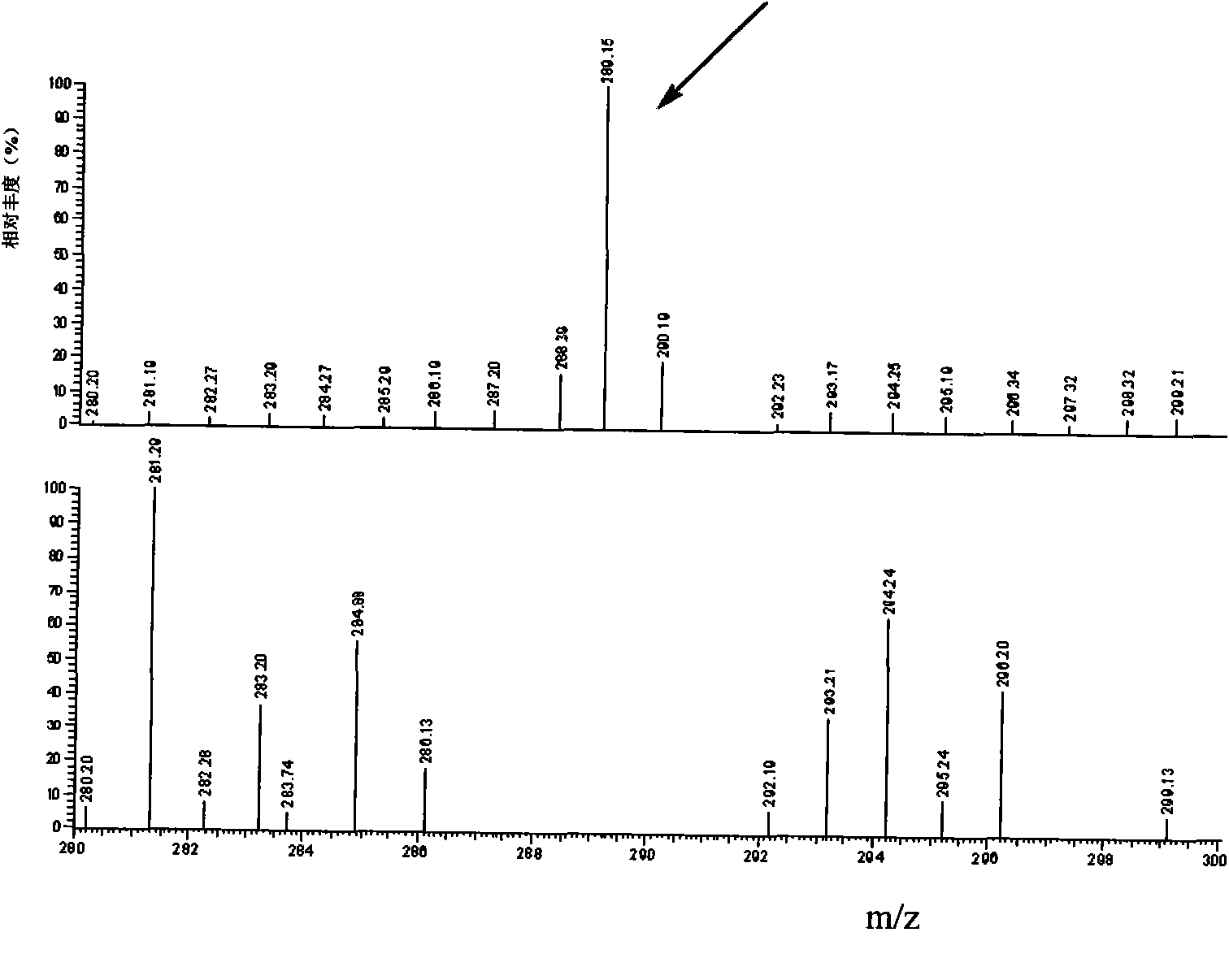
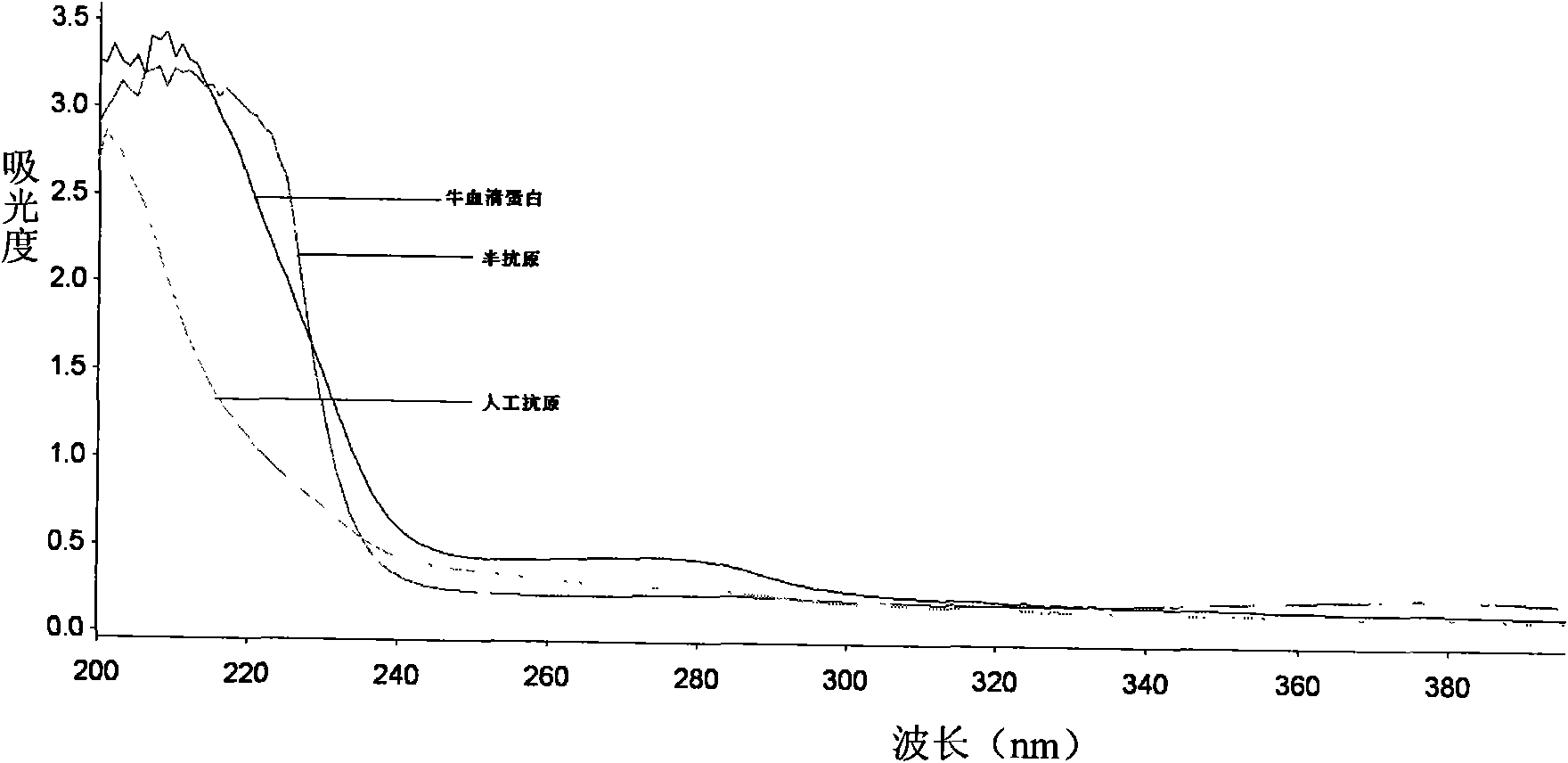
[0033] 1. Synthesis and identification of melamine hapten
[0034] 1. Synthesis of melamine hapten
[0035] Weigh 0.430 g (3 mmol) of 2-chloro-4,6-diamino-1,3,5-triazine (CAAT) and suspend in Na 2 SO4 In the 10mL dehydrated ethanol extraction of treatment, add 0.5g (3mmol) p-aminophenylbutyric acid (2-chloro-4,6-diamino-1,3,5-triazine and p-aminophenylbutyric acid feed intake The molar ratio is 1:1), and KOH was added to make the final concentration of KOH 85%. The water bath was refluxed at 75° C. for 24 hours, and 0.37 g p-aminophenylbutyric acid (2.0 mmol) and 85% KOH (180 mg, 2.7 mmol) were added at 16 hours and 19 hours, respectively. The product was filtered and distilled under reduced pressure to obtain an oily substance. The product was dissolved in 30 mL of 5% sodium carbonate solution, washed twice with ...
Embodiment 2
[0046] Embodiment 2, preparation and identification of melamine hapten and melamine antigen
[0047] 1. Synthesis and identification of melamine hapten
[0048] 1. Synthesis of melamine hapten
[0049] Weigh 2-chloro-4,6-diamino-1,3,5-triazine (CAAT) and suspend in Na 2 SO4 In the 10mL dehydrated ethanol extraction of treatment, add p-aminophenylbutyric acid (2-chloro-4,6-diamino-1,3,5-triazine and p-aminophenylbutyric acid have a molar ratio of 1:2 ), adding KOH so that the final concentration of KOH is 85%. The water bath was refluxed at 70° C. for 26 hours, and 0.37 g p-aminophenylbutyric acid (2.0 mmol) and 85% KOH (180 mg, 2.7 mmol) were added at 16 hours and 19 hours, respectively. The product was filtered and distilled under reduced pressure to obtain an oily substance. The product was dissolved in 30 mL of 5% sodium carbonate solution, washed twice with 10 mL of dichloromethane, the pH of the aqueous phase was adjusted to 1 with concentrated hydrochloric acid, the ...
Embodiment 3
[0057] Embodiment 3, preparation and identification of melamine hapten and melamine antigen
[0058] 1. Synthesis and identification of melamine hapten
[0059] 1. Synthesis of melamine hapten
[0060] Weigh 2-chloro-4,6-diamino-1,3,5-triazine (CAAT) and suspend in Na 2 SO4 In the 10mL dehydrated ethanol extraction of treatment, add p-aminophenylbutyric acid (2-chloro-4,6-diamino-1,3,5-triazine and p-aminophenylbutyric acid have a molar ratio of 1:3 ), adding KOH so that the final concentration of KOH is 85%. The water bath was refluxed at 80° C. for 30 hours, and 0.37 g p-aminobenzenebutyric acid (2.0 mmol) and 85% KOH (180 mg, 2.7 mmol) were added at 16 hours and 19 hours, respectively. The product was filtered and distilled under reduced pressure to obtain an oily substance. The product was dissolved in 30 mL of 5% sodium carbonate solution, washed twice with 10 mL of dichloromethane, the pH of the aqueous phase was adjusted to 1 with concentrated hydrochloric acid, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com