Degradable and nontoxic medical polyurethane material and preparation method thereof
A technology of polyurethane material and lysine methyl ester, applied in the field of biomedical materials, can solve problems such as difficult degradation or toxic degradation products, and achieve the effects of improving mechanical properties and degradation properties, low price and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
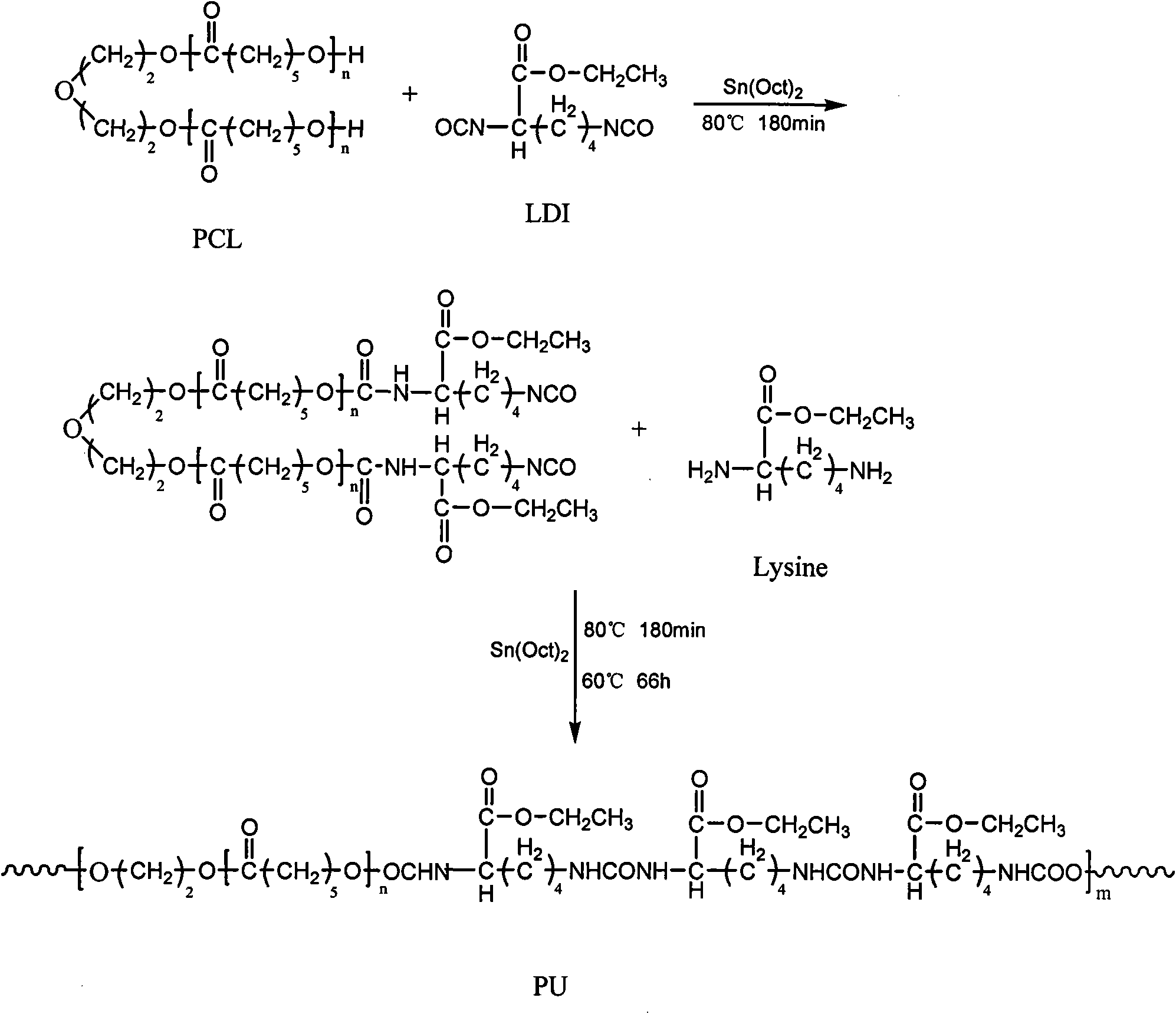
[0035] The preparation of polyurethane (PU-Lys1) is fed according to the molar ratio of PCL:Lysine-E:LDI-E=0.9:0.1:1.1.
[0036] First 20.00 grams of poly(ε-caprolactone) diols with a number average molecular weight of 2000 and 2.76 grams of LDI-E were added to a three-necked flask, and 10 milliliters of DMF and 0.1% of the catalyst stannous octoate were added, at 80°C under nitrogen protection React for 3 hours. Then add 0.28 g of L-lysine ethyl ester dihydrochloride, add 0.34 g of triethylamine after mixing for 15 minutes, react at 80° C. for 3 hours, then drop the temperature to 60° C. and continue the reaction for 66 hours. After the reaction is completed, the N, N-dimethylformamide solution of polyurethane is poured into distilled water. Precipitation occurs, and then filtered. After filtering, the filter cake is washed with deionized water to remove triethylamine hydrochloride, and then filtered. The cake was vacuum-dried at 80°C for 24 hours to obtain polyurethane. Po...
Embodiment 2
[0043] The preparation of polyurethane (PU-Lys2) is fed according to the molar ratio of PCL:Lysine-E:LDI-E=0.7:0.3:1.1.
[0044] First 20.00 grams of poly(ε-caprolactone) diols with a number average molecular weight of 2000 and 3.55 grams of LDI-E were added to a three-necked flask, and 10 milliliters of DMF and 0.1% of the catalyst stannous octoate were added, at 80°C under nitrogen protection React for 3 hours. Then add 1.06 g of L-lysine ethyl ester dihydrochloride, add 1.30 g of triethylamine after mixing for 15 minutes, react at 80° C. for 3 hours, then drop the temperature to 60° C. and continue the reaction for 66 hours. After the reaction is completed, the N, N-dimethylformamide solution of polyurethane is poured into distilled water. Precipitation occurs, and then filtered. After filtering, the filter cake is washed with deionized water to remove triethylamine hydrochloride, and then filtered. The cake was vacuum-dried at 80°C for 24 hours to obtain polyurethane. Po...
Embodiment 3
[0051] The preparation of polyurethane (PU-Lys3) is fed according to the molar ratio of PCL:Lysine-E:LDI-E=0.5:0.5:1.1.
[0052] First 20 grams of poly(ε-caprolactone) glycols and 4.97 grams of LDI-E with a number-average molecular weight of 2000 were added to a three-necked flask, and 10 milliliters of DMF and 0.1% catalyst stannous octoate were added, at 80°C under nitrogen protection React for 3 hours. Then add 2.47 grams of L-lysine ethyl ester dihydrochloride, add 3.03 grams of triethylamine after mixing for 15 minutes, react at 80° C. for 3 hours, then drop the temperature to 60° C. and continue the reaction for 66 hours. After the reaction is completed, the N, N-dimethylformamide solution of polyurethane is poured into petroleum ether. Precipitation occurs, and then filtered. After filtering, the filter cake is washed with deionized water to remove triethylamine hydrochloride, and then filtered. The filter cake was vacuum-dried at 80°C for 24 hours to obtain polyuretha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| breaking strength | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
| breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com