Alpha-galactosidase and coding gene thereof
A technology for galactosidase and coding gene, which is applied in the field of α-galactosidase and its coding gene, and can solve the problem of low similarity, low efficiency and limited means of separating α-galactosidase. and other problems to achieve the effect of good commercial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, the acquisition of α-galactosidase coding gene
[0057] 1. Obtain the partial sequence of the enzyme-encoding gene by designing degenerate primers
[0058] Aspergillus pylorus belongs to the genus Aspergillus, so search for the existing α-galactosidase gene protein sequence derived from Aspergillus, using the multiple sequence alignment program
[0059] BLOCKS[http: / / blocks.fhcrc.org / block / make_blocks.html] analysis found two conservative sequences W(G / E)IDYLKYD and HPAEYWSGP. Based on these two conserved sequences, a pair of degenerate primers were designed to amplify the partial α-galactosidase sequence from the genomic DNA of Aspergillus pyrogenus by PCR.
[0060] Degenerate primers were designed based on the two conserved regions and synthesized using a DNA synthesizer. PCR amplification was carried out using the genomic DNA of Aspergillusustus as a template. Except for degenerate primers and genomic DNA templates, other reagents used for PCR amplif...
Embodiment 2
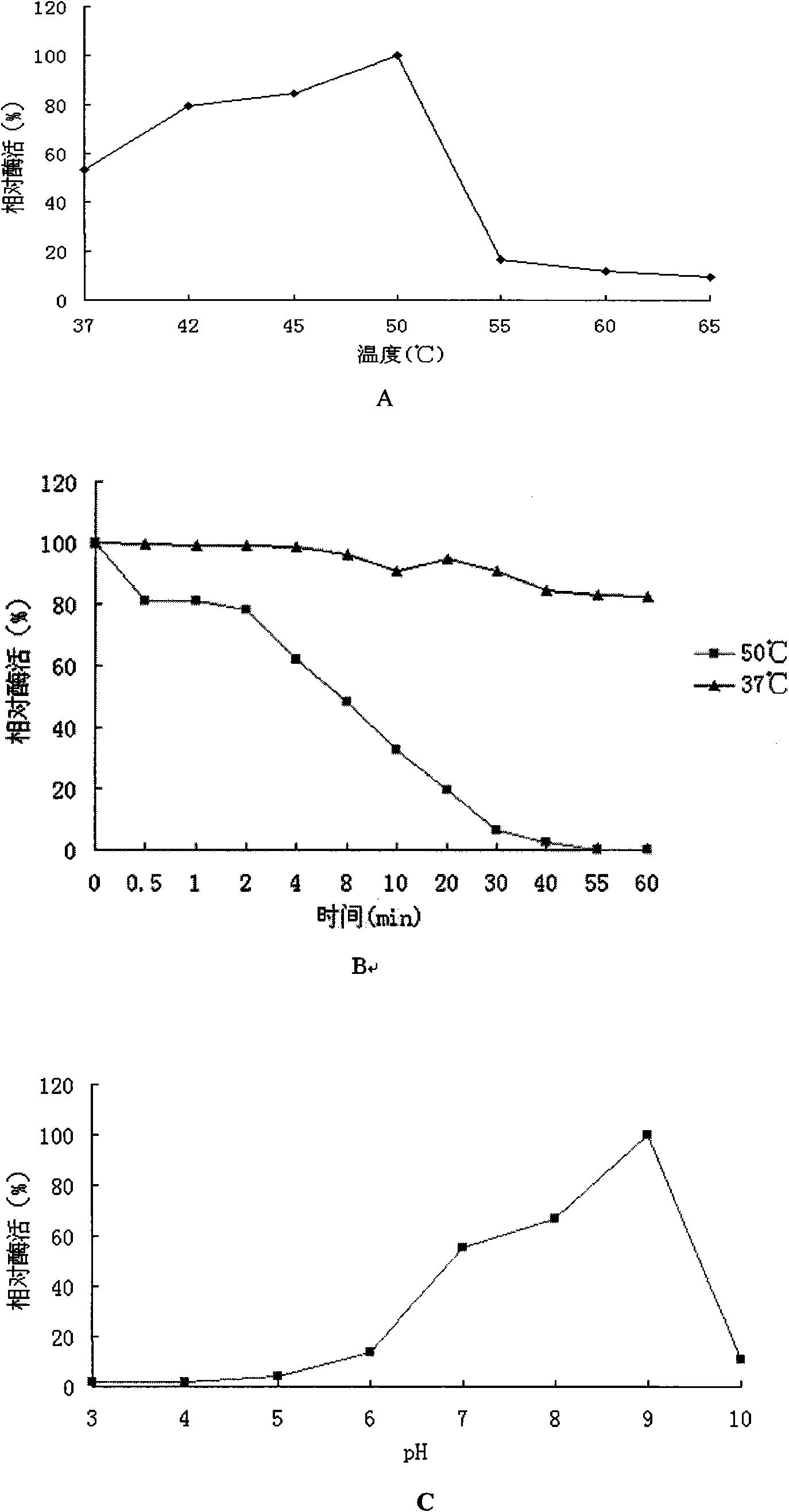
[0184] Embodiment 2, genetic engineering expression obtains α-galactosidase and the enzymatic property analysis of this enzyme
[0185] 1. Genetic engineering expression to obtain α-galactosidase
[0186] 1. Preparation of α-galactosidase coding gene
[0187] With QIAGEN RNeasy Plant Mini kit (RNeasy Plant Mini Kit, Cat.no.74903), extract the total RNA of Aspergillus ustus (CGMCC NO.3.3534); Utilize MBI reverse transcription kit (Fermentas: RevertAid TM First Strand cDNA Synthesis Kit, #K1621) was reverse transcribed to obtain cDNA; using cDNA as a template, PCR amplification was performed with the following primers:
[0188] JYF: (ECOR I) GAATTC ATGTTACTTTCAGTGATCGTCAT (sequence 8)
[0189] JYR: (Not I) GCGGCCGC TTAGTTGCTATAATATGTCCCAT (sequence 9)
[0190] The PCR amplification product was sequenced and identified, and the sequencing result showed that the sequence of the PCR product was shown in SEQID NO.2 in the sequence listing.
[0191] 2. Construction of recomb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com