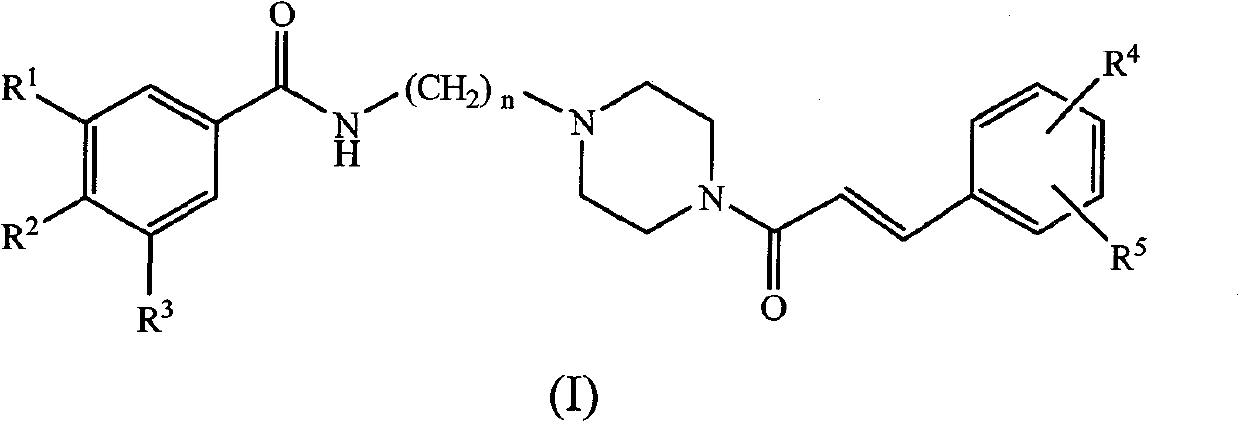
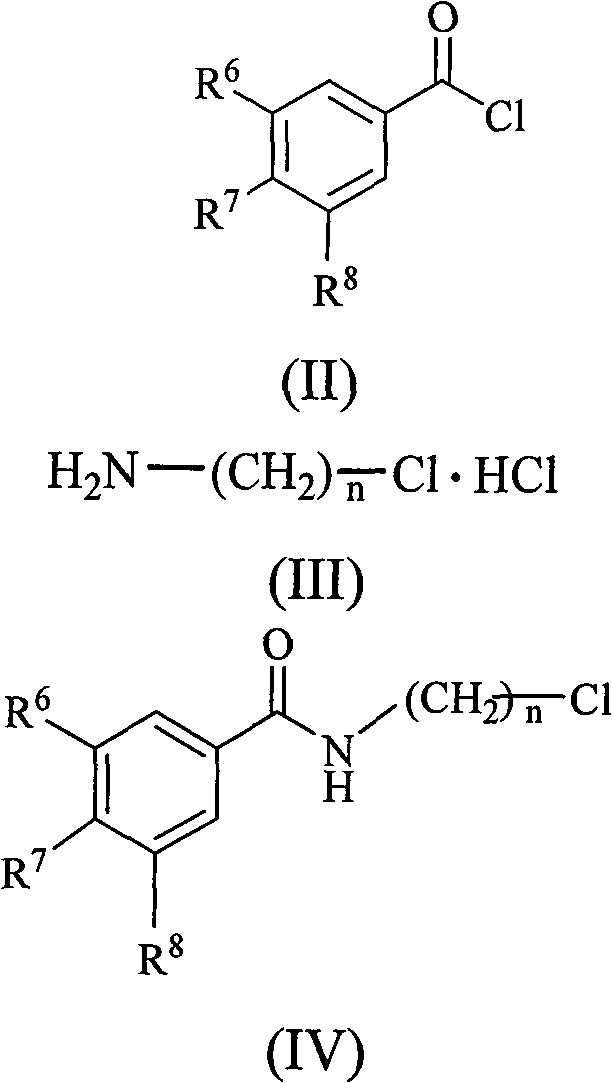
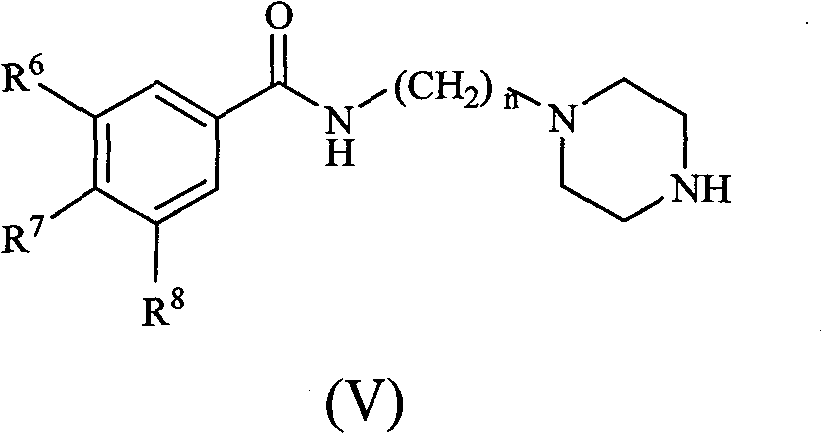
Substituted benzene propenyl piperazinyl alkyl polyhydric benzamide compound and preparation method and application thereof
A compound and alkyl technology, which is applied in the field of preparation of HIV-1 integrase inhibitors, can solve the problems of drug resistance, central nervous disorder, multi-drug resistance, etc., and achieve the effect of mild reaction conditions and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of (E)-N-(3-(4-phenylacryloylpiperazin-1-yl)propanyl)-3,4,5-trihydroxybenzamide
[0042] (a) Add 0.03mol of 3-chloropropylamine hydrochloride into the three-necked flask, then add 30mL of anhydrous-treated dichloromethane, and add dropwise to the dichloromethane solution of 3-chloropropylamine hydrochloride 10mL of triethylamine solution, stirred and reacted for half an hour, then added dropwise 0.01mol of 3,4,5-trimethoxybenzoyl chloride in dichloromethane solution at 0°C, after the dropwise addition was completed, remove the ice bath, React overnight at room temperature. The reaction system was washed with 30mL saturated NaHCO 3 solution, 30mL of 2mol / L hydrochloric acid solution and 30mL of saturated saline solution, the organic phase was separated and dried with anhydrous sodium sulfate, and vacuum precipitation can obtain N-3-chloropropyl-3,4,5-trimethyl Oxybenzamide, yield: 88%.
[0043] (b) Preparation of N-3-(piperazin-1-yl)propyl-3,4,5-trimethoxy...
Embodiment 2
[0063] Preparation of (E)-N-(3-(4-p-methylbenzoylpiperazin-1-yl)propanyl)-3,4,5-trihydroxybenzamide
[0064] (a), (b) steps are with embodiment 1
[0065] (c) Preparation of (E)-N-(3-(4-p-toluacryloylpiperazin-1-yl)-propanyl)-3,4,5-trimethoxybenzamide
[0066]
[0067] Add 10 mL of thionyl chloride to 4.0 mmol of p-methylcinnamic acid, reflux for 4 to 5 hours, remove excess thionyl chloride under reduced pressure, and add 15 mL of dichloromethane after cooling for later use.
[0068] 3mmol of NaHCO 3 Add N-3-(piperazin-1-yl)propyl-3,4,5-trimethoxybenzamide (3mmol) in dichloromethane solution, and dichloride of p-toluacryloyl chloride The methane solution was slowly added dropwise to the stirred solution at 0°C, and after 1 hour, the ice bath was removed and left to stand overnight, and then 30 mL of saturated NaHCO 3 solution, 30mL of 2molL -1 Hydrochloric acid and 30 mL of saturated saline solution, the organic phase was separated with a separatory funnel, dried over a...
Embodiment 3
[0080] Preparation of (E)-N-(3-(4-p-chlorophenylacryloylpiperazin-1-yl)propanyl)-3,4,5-trihydroxybenzamide
[0081] (a), (b) steps are with embodiment 1
[0082] (c) Preparation of (E)-N-(3-(4-p-chlorophenylacryloylpiperazin-1-yl)-propanyl)-3,4,5-trimethoxybenzamide
[0083]
[0084] Add 10 mL of thionyl chloride to 3.8 mmol of p-chlorocinnamic acid, reflux for 4 to 5 hours, remove excess thionyl chloride under reduced pressure, and add 15 mL of dichloromethane after cooling for later use.
[0085] 3mmol of NaHCO 3 Add N-3-(piperazin-1-yl)propyl-3,4,5-trimethoxybenzamide (3mmol) in dichloromethane solution, and then add p-chlorophenylacryloyl chloride in dichloromethane The solution was slowly added dropwise to the stirred solution at 0°C, and after 1 hour, the ice bath was removed and left to stand overnight, and then 30 mL of saturated NaHCO 3 solution, 30mL of 2molL -1 Hydrochloric acid and 30 mL of saturated saline solution, the organic phase was separated with a se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com