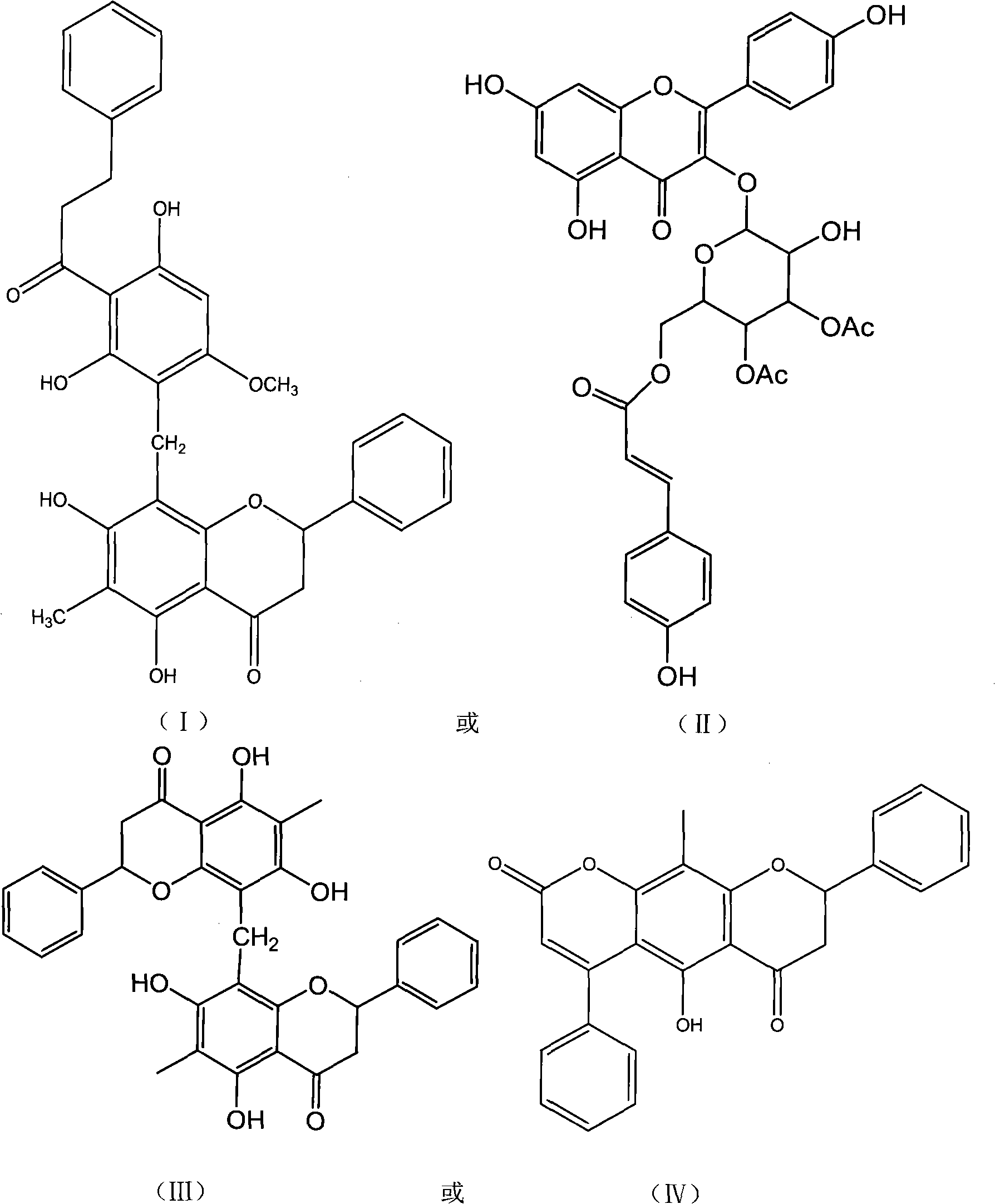
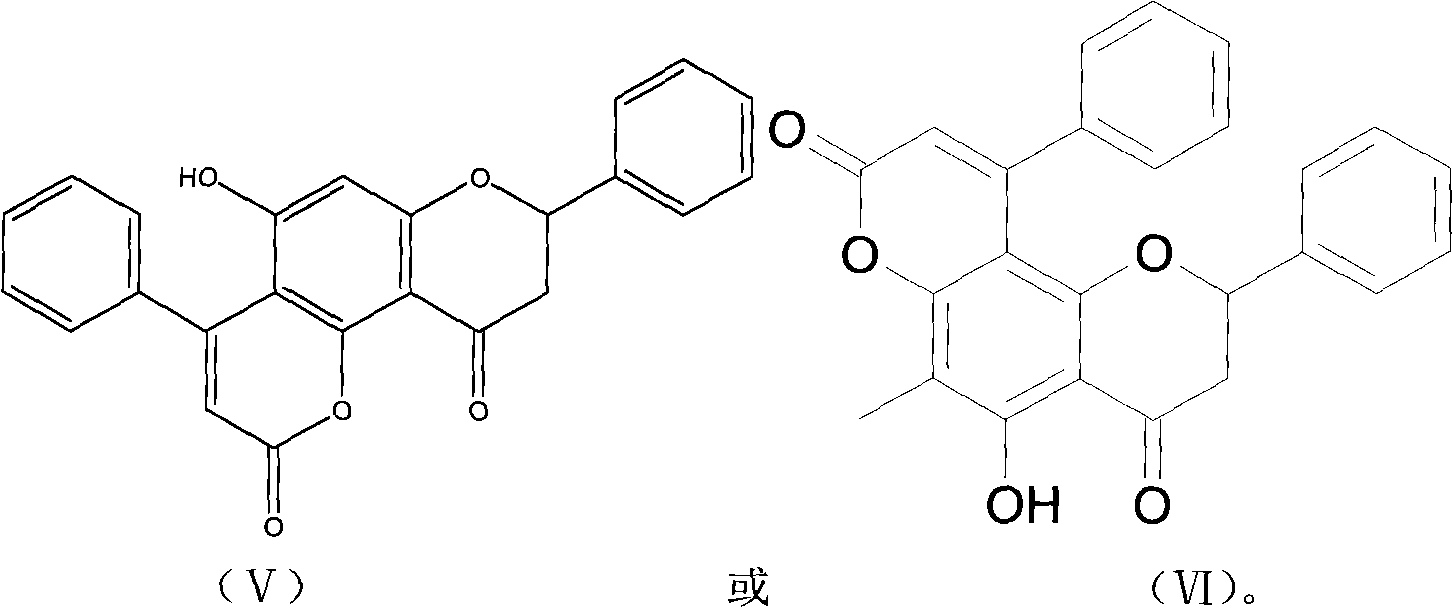
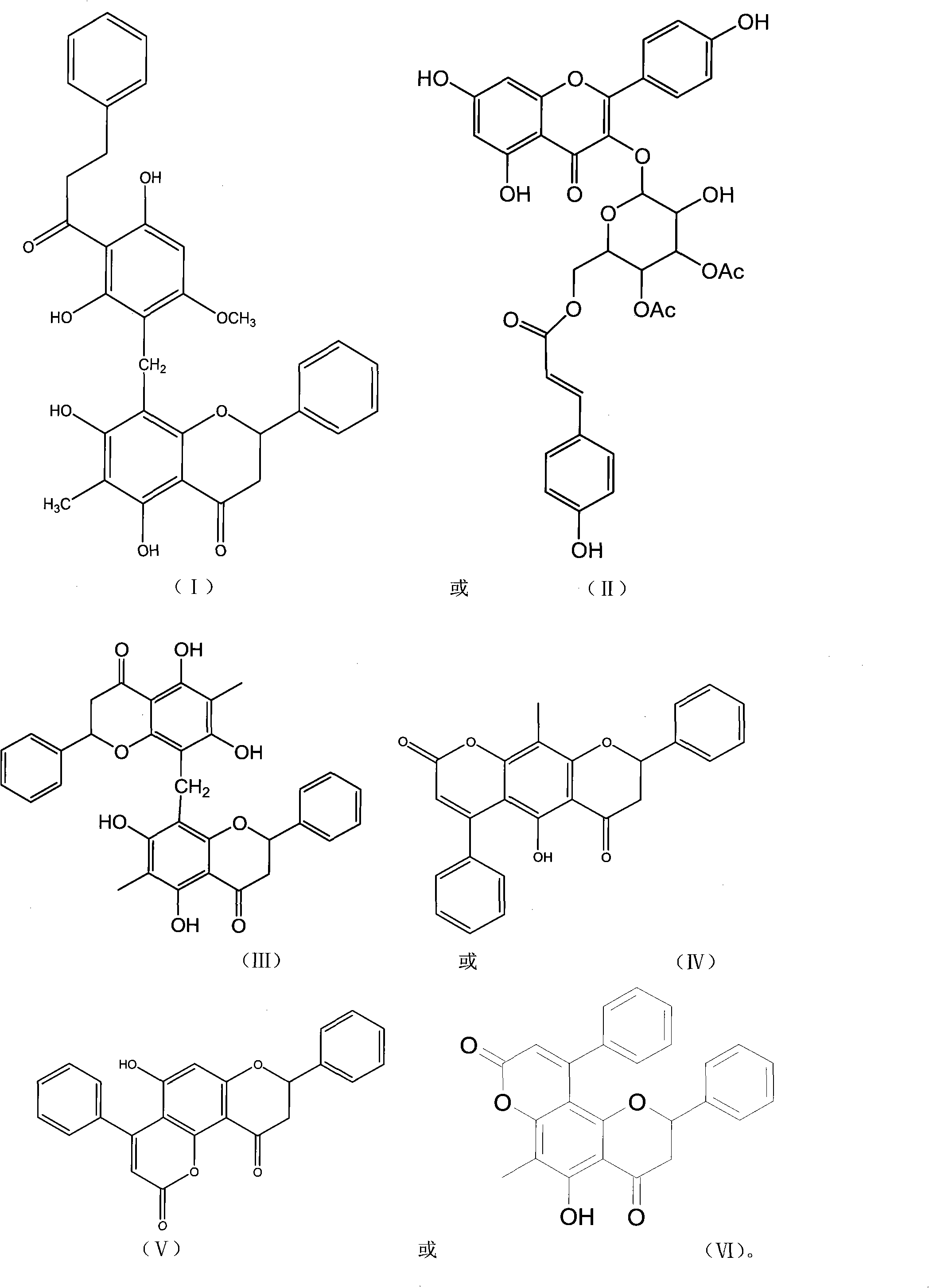
Pronephrium megacuspe compound, preparation method thereof and application
A compound, the technology of crescent fern, applied in the field of chemistry, achieves the effect of simple preparation method, low preparation cost, and inhibition of cancer cell activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of Crescentin A, Crescentin B, Crescentin C, Crescentin D, Crescentin E and Crescentin F
[0034] (1) Get 7.5Kg of the whole herb of Crescent fern, dry in the shade, pulverize, extract by cold soaking in 80ml of 95% methanol for 3 times, each time for 3 days, combine the filtrates extracted by cold soaking for 3 times, and concentrate under reduced pressure to obtain The extract is about 800g;
[0035] (2) Suspend the extract in 1 liter of water, extract 3 times with 1.5 liter, 1 liter, and 1 liter of ethyl acetate respectively, combine the extracts for recovery under reduced pressure, and obtain 230 g of ethyl acetate partial extract;
[0036] (3) The ethyl acetate extraction part obtains 4 fractions after 300 mesh silica gel atmospheric pressure column chromatography, column chromatography condition: trichloromethane: methanol=1: 0 to 1: 1; 4 fractions are trichloromethane respectively :methanol=1:0, chloroform:methanol=20:1, chloroform:methanol=10:1, chl...
Embodiment 2
[0051] Embodiment 2 The compound prepared by the present invention has the experiment of biological activity to insect
[0052] The method for measuring the antifeedant activity against the 3rd instar larvae of Ostrinia sativa: the compound prepared by the method of the present invention is dissolved in acetone, and prepared into a mother solution with a concentration of 1000 μg / mL. The mother liquor was prepared with acetone+water to make a drug solution with a concentration of 50 μg / mL, and the final concentration of acetone in the drug was 30%. Using the fresh corn heart leaf method, the diameter of the leaf disk is 1cm, and the leaf disk is soaked with the prepared medicinal solution for 3 seconds, and after the solvent is evaporated, put it into a petri dish lined with wet filter paper. One 3rd instar larva of Ostrinia officinalis starved for 4 hours, each treatment was repeated 10 times, and the leaves were replaced with new pesticides after checking the experimental res...
Embodiment 3
[0060] Embodiment 3 The compound of the present invention has the experiment of cytotoxicity
[0061] Preparation of medicinal solution: Dissolve the test compound prepared by the present invention with a small amount of acetone or dimethyl sulfoxide (DMSO) to make mother liquor, filter and sterilize with a 0.22 μm microporous membrane on the ultra-clean workbench, and use In the medium containing 1% DMSO, the reagents were formulated to the concentration required for the test, the final concentration of acetone was 2.5%, and the final concentration of DMSO was 1%. The medium containing 1% DMSO or 2.5% acetone was used as a control.
[0062] In vitro cytotoxicity test method: MTT method is used. Inoculate SL cells in logarithmic growth phase, human liver cancer cells (SMMC-7721), and human breast cancer cells (MCF-7) into 96-well culture plates, add 100 μL to each well, and culture for 24 hours. Add 100 μL of the corresponding concentration of drug solution to each well, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com