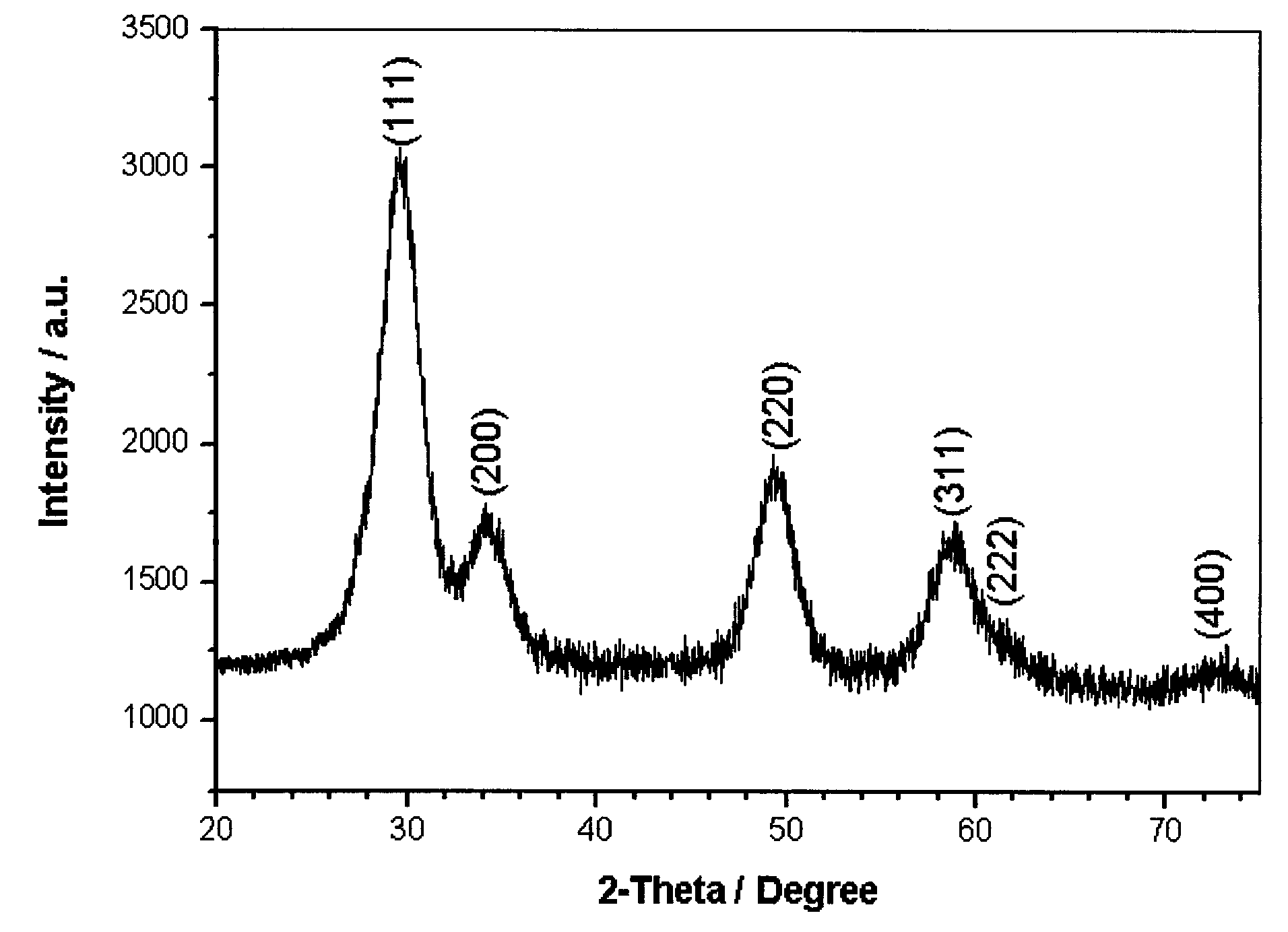
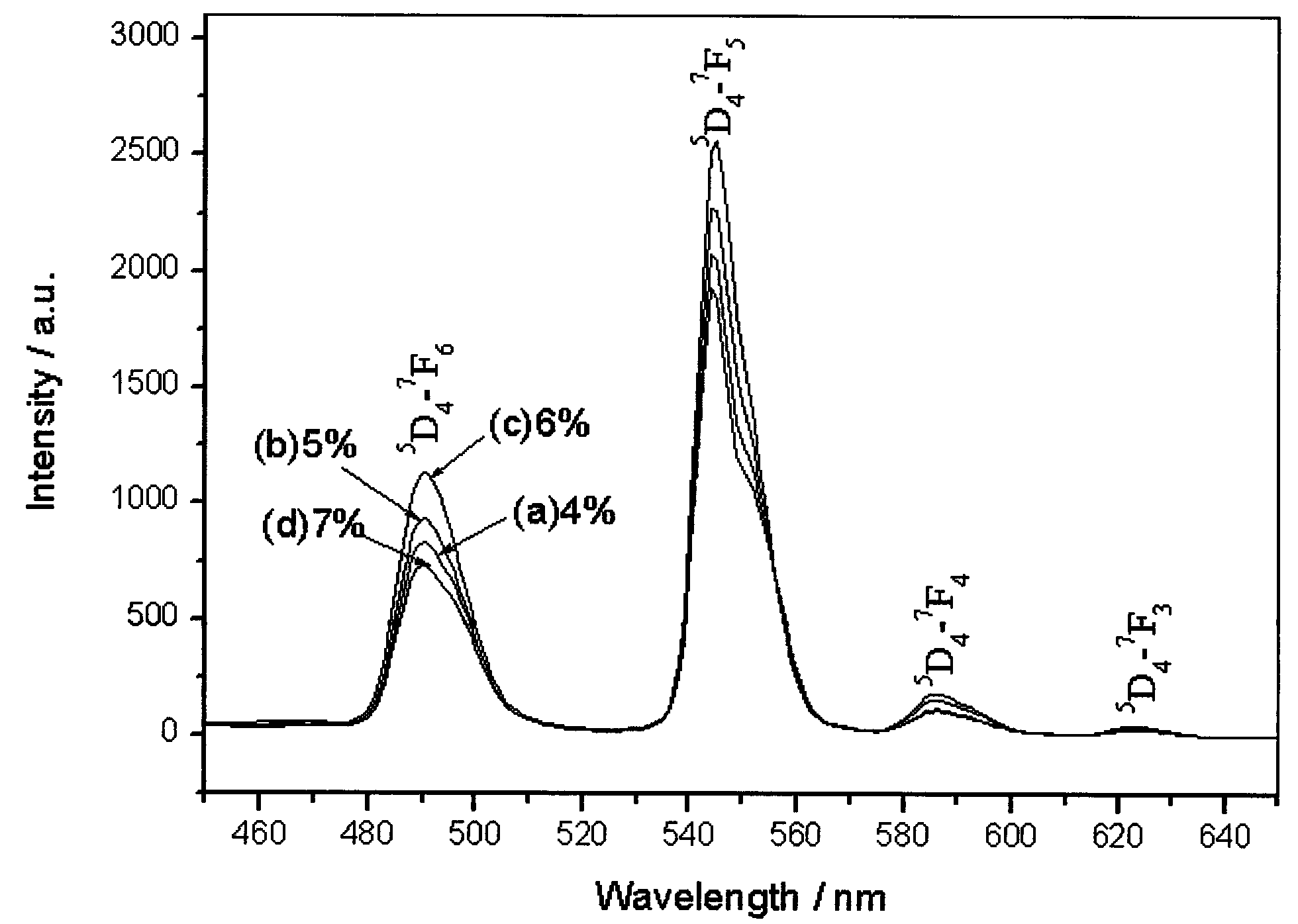
Preparation method of dysprosium zirconate terbium-doped green luminescent material
A luminescent material, dysprosium zirconate technology, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of high synthesis temperature, less research, single preparation method, etc., and achieves good crystallinity, mild reaction conditions, and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Get 1.3695 grams of dysprosium nitrate (Dy(NO 3 ) 3 ·6H 2 O), 0.0566 grams of terbium nitrate (Tb(NO 3 ) 3 ·6H 2 O) be dissolved in 40 milliliters of deionized water, the molar concentrations of dysprosium nitrate and terbium nitrate are respectively 0.0750 mol / liter and 0.0031 mol / liter, and stir for 15 minutes; get zirconium oxychloride (ZrOCl 2 ·8H 2 O) 1.0069 grams were dissolved in 40 milliliters of deionized water, the molar concentration of zirconium oxychloride was 0.0781 mol / liter, and stirred for 15 minutes. The zirconium oxychloride solution was dropped dropwise into the above mixed solution of dysprosium nitrate and terbium nitrate, and stirred for 15 minutes. 1 mol / L sodium hydroxide solution was added dropwise to the above mixed solution to adjust the pH value of the solution to 8.0, and the stirring was continued for 0.5 hour. Put the above prepared solution into a polytetrafluoroethylene-lined autoclave with a filling degree of 80% and a liner vol...
Embodiment 2
[0018] Get 2.2825 grams of dysprosium nitrate (Dy(NO 3 ) 3 ·6H 2 O), 0.1192 grams of terbium nitrate (Tb(NO 3 ) 3 ·6H 2 O) be dissolved in 40 milliliters of deionized water, the molar concentrations of dysprosium nitrate and terbium nitrate are respectively 0.125 mol / liter and 0.0066 mol / liter, and stir for 20 minutes; take zirconium oxychloride (ZrOCl 2 ·8H 2 O) 1.6959 grams were dissolved in 40 milliliters of deionized water, the molar concentration of zirconium oxychloride was 0.1316 mol / liter, and stirred for 15 minutes. The zirconium oxychloride solution was dropped dropwise into the above mixed solution of dysprosium nitrate and terbium nitrate, and stirred for 20 minutes. 1 mol / L sodium hydroxide solution was added dropwise to the above mixed solution to adjust the pH value of the solution to 8.5, and the stirring was continued for 0.5 hour. Put the above prepared solution into a polytetrafluoroethylene-lined autoclave with a filling degree of 80% and a liner vol...
Embodiment 3
[0020] Get 4.565 grams of dysprosium nitrate (Dy(NO 3 ) 3 ·6H 2 O), 0.2891 grams of terbium nitrate (Tb(NO 3 ) 3 ·6H 2 O) be dissolved in 40 milliliters of deionized water, the molar concentrations of dysprosium nitrate and terbium nitrate are respectively 0.25 mol / liter and 0.016 mol / liter, and stir for 20 minutes; take zirconium oxychloride (ZrOCl 2 ·8H 2 O) 3.4279 grams were dissolved in 40 milliliters of deionized water, the molar concentration of zirconium oxychloride was 0.266 mol / liter, and stirred for 15 minutes. The zirconium oxychloride solution was dropped dropwise into the above mixed solution of dysprosium nitrate and terbium nitrate, and stirred for 20 minutes. 1 mol / L sodium hydroxide solution was added dropwise to the above mixed solution to adjust the pH value of the solution to 9.0, and the stirring was continued for 0.5 hour. Put the above prepared solution into a polytetrafluoroethylene-lined autoclave with a filling degree of 80% and a liner volume ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com