Application of alkannin derivant
A derivative, shikonin technology, applied in the field of application of shikonin derivatives, can solve problems such as no discovery, modification and optimization, and unclear anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 And preparation of shikonin derivatives
[0029] Weigh 20 kg of Liaoning Lithospermum (commercially available), grind and pass through an 80-mesh sieve, collect the comfrey powder obtained after sieving, and perform percolation extraction with 95% ethanol at room temperature until the extract is nearly colorless.
[0030] The extracts were combined and concentrated under reduced pressure until the solution became purple-red, and about 250 g of crude product of purple-brown oil was obtained.
[0031] After filtering to remove the insoluble matter, add concentrated HCl to acidify the solution until the solution turns from blue to purple-red, at which time a large amount of precipitation is formed, and let it stand overnight.
[0032] Dissolve the crude product with chloroform, add 17 grams of 200-300 mesh silica gel per 50 milliliters, and stir evenly on the column. As the eluent, petroleum ether (60-90° C.)-acetone and petroleum ether (1:1)-acetone-chloroform (1:1) we...
Embodiment 2
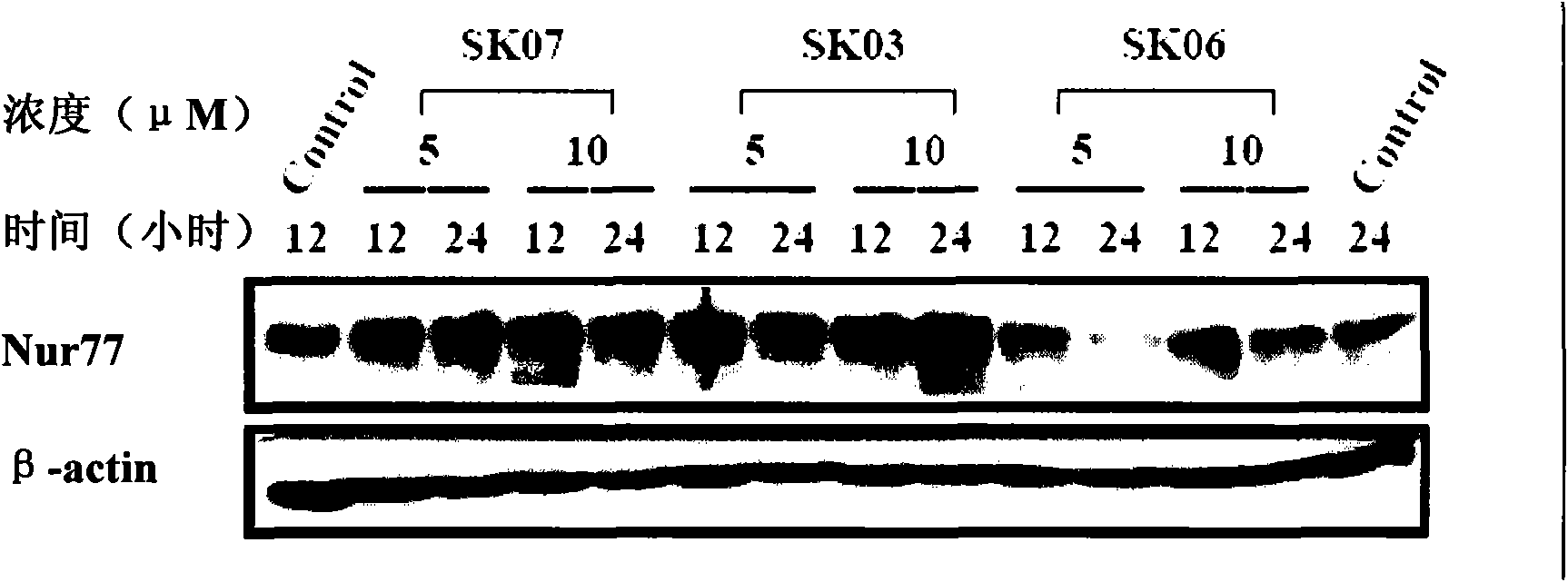
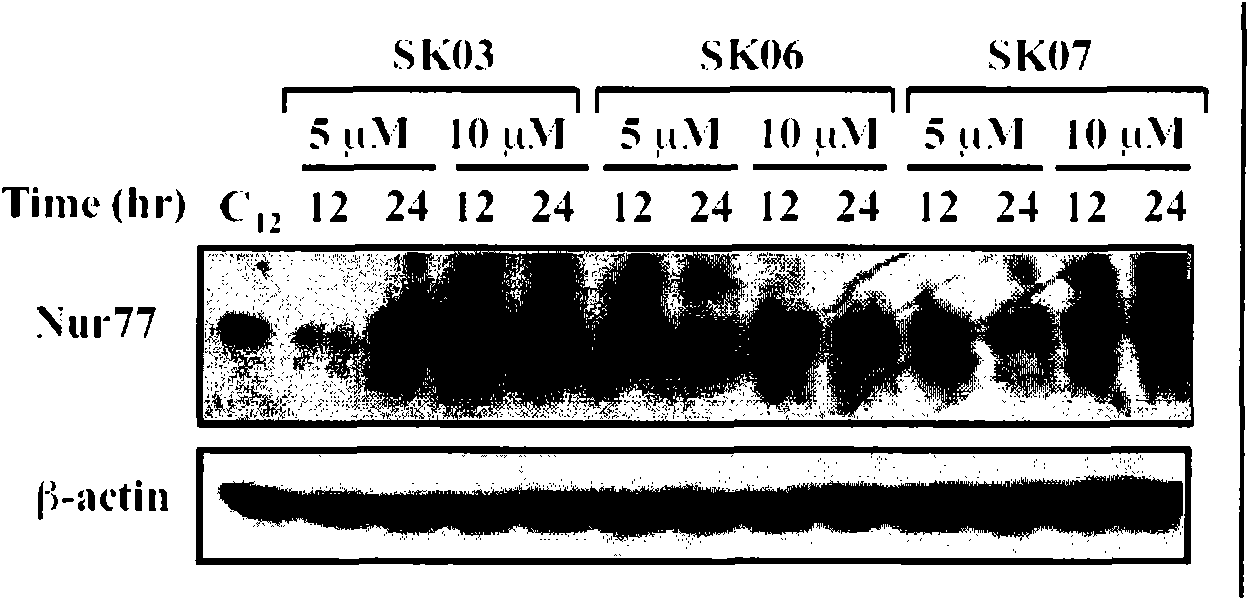
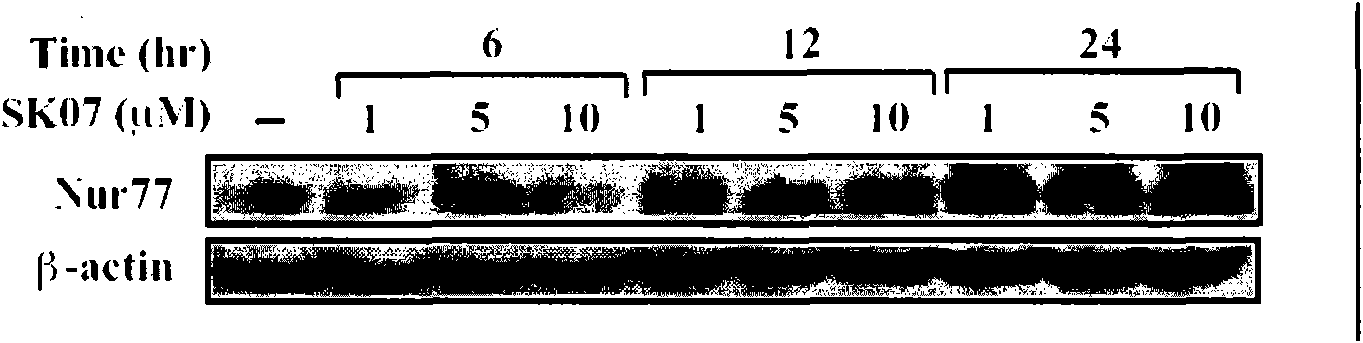
[0036] Example 2 , Shikonin derivatives inducing the expression of Nur77
[0037] 2.1, cell culture
[0038] Select lung cancer cell NIH-H460 (ATCC, HTB-177) and cervical cancer cell HeLa (ATCC, CCL-2), use RPM1640 medium (purchased from Hyclone), culture in a 24-well plate at 37℃ and 5% carbon dioxide incubator In the tissue culture plate, the medium was changed after 24 hours, and the drug (without serum) was treated after 16 hours of starvation. Shikonin derivatives are dissolved in DMSO (final concentration of DMSO <0.1%), and the control group is treated with the same concentration of DMSO. The specific treatment is as follows:
[0039] (1) Control group (DMSO)
[0040] (2) SK03 group
[0041] (3) SK06 group
[0042] (4) SK07 group
[0043] The concentrations of SK03, SK06, and SK07 were all 5 or 10 μM, and the treatment time was 12 or 24 hours. NIH-H460 cells and HeLa cells without any treatment were used as controls. In order to confirm the time-effect and dose-effect relations...
Embodiment 3
[0064] Example 3 , Shikonin derivatives induce tumor cell apoptosis
[0065] 3.1 Shikonin derivatives induce NIH-H460 cell apoptosis
[0066] Select lung cancer cells NIH-H460 (ATCC, HTB-177), use RPM1640 medium (purchased from Hyclone), culture in a 24-well tissue culture plate at 37°C and 5% carbon dioxide incubator, change the medium and proceed after 24 hours Dosing (without serum) treatment. Shikonin derivatives are dissolved in DMSO (final concentration of DMSO <0.1%), and the control group is treated with the same concentration of DMSO. The specific treatment is as follows:
[0067] (1) Control group (DMSO)
[0068] (2) SK03 group
[0069] (3) SK06 group
[0070] (4) SK07 group
[0071] The total volume of each well solution in the reaction plate is 1ml, and the concentrations of SK03, SK06 and SK07 are all 5μM.
[0072] After 24 hours of treatment, the cells were digested with 0.5% trypsin, washed with 0.1M PBS, fixed with 4% paraformaldehyde, stained with 1μg / ml DAPI, and obser...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com