CO sulfur tolerant shift catalyst and preparation method thereof
A sulfur-resistant conversion and catalyst technology, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of failure to reach the second type of catalyst, adverse effects of subsequent stages, easy loss of alkali metal, etc. problems, to achieve the effect of increasing interaction, improving strength and stability, and highlighting substantive features
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Phase inversion of natural magnesite ore into active magnesia ore powder
[0045] Take a natural magnesite ore in Laizhou, Shandong, its composition and phase: MgO: 45.35%, CaO: 0.60%, SiO 2 : 0.2%, Al 2 o 3 : 0.6%, Fe 2 o 3 : 0.4%, CO 2 : ~46.85%. The phase composition of magnesite was analyzed by x-ray diffractometer, the main component of the ore is MgCO 3 . Pulverize the ore, put it into a muffle furnace, raise the temperature to 800°C, and heat the phase inversion at 800°C for 2 hours, then naturally cool down to obtain active magnesia ore powder; the composition of active magnesia: MgO: 92.6%, CaO: 1.2%, SiO 2 : 0.4%, Al 2 o 3 : 0.9%, Fe 2 o 3 : 0.8%. The active magnesia ore powder is analyzed by X-ray diffractometer, and its main component is MgO.
Embodiment 2
[0046] Example 2: Phase inversion of natural magnesite ore into active magnesia ore powder
[0047] Take a natural magnesite ore in Haicheng, Liaoning, its composition and phase: MgO: 46.62%, CaO: 0.86%, SiO2: 0.2%, Al2O3: 0.3%, Fe2O3: 0.3%, CO2: ~46.4%. The phase composition of magnesite was analyzed by X-ray diffractometer, and the main component of the ore was MgCO3. Pulverize the ore, put it into a muffle furnace, raise the temperature to 800°C, keep warm at 800°C for 2 hours, and then cool down naturally to obtain active magnesia ore powder; the composition of active magnesia: MgO: 93.1%, CaO: 1.5%, SiO2: 0.3%, Al2O3: 0.5%, Fe2O3: 0.5%. The active magnesia ore powder is analyzed by X-ray diffractometer, and its main component is MgO.
Embodiment 3
[0048] Example 3 Preparation of Sulfur Tolerant Shift Catalyst Using Activated Magnesia Powder
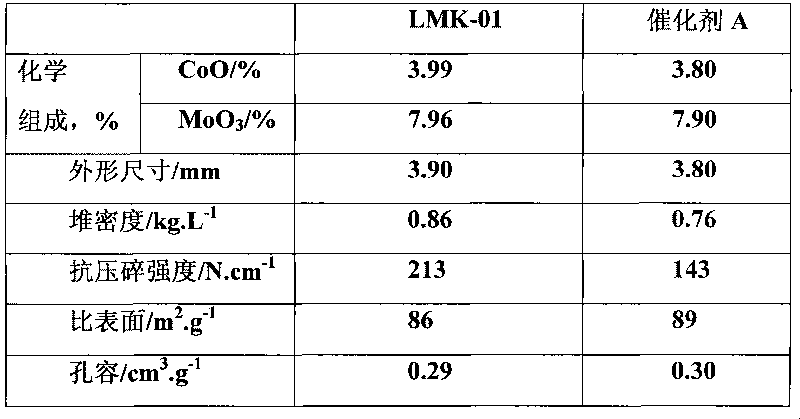
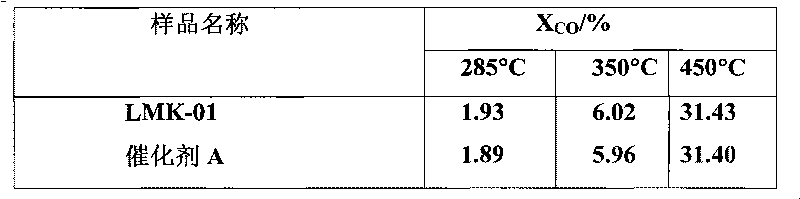
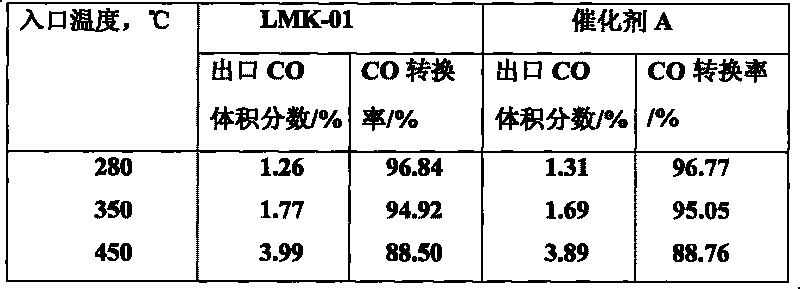
[0049] Add 220 g of the active magnesia powder obtained in Example 1, 760 g of pseudo-boehmite and 20 g of urea into a ball mill, mix and mill for 1 hour to obtain a powdery mixture, which is kneaded together with 30 g of scallop powder Dry mix in the machine for 10 minutes, add 95g ammonium paramolybdate, 150g cobalt nitrate and 30g citric acid aqueous solution and knead for 60 minutes. . The finished catalyst can be obtained, the number is LMK-01. Catalyst test results: The prepared LMK-01 catalyst and commercial catalyst A were tested according to the normal catalyst physical and chemical performance test method and the above-mentioned activity evaluation method. The test results are shown in Table 1, Table 2 and Table 3.
[0050] Directly use natural magnesite ore powder to directly produce sulfur-resistant shift catalyst, the physical and chemical properties and catalytic act...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com