Magnetic nano hydroxyapatite adsorbent, preparation and application thereof
A technology of nano-hydroxyapatite and hydroxyapatite, which is applied in the direction of adsorption of water/sewage treatment, other chemical processes, chemical instruments and methods, etc., to achieve the effects of simple preparation, high adsorption efficiency and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
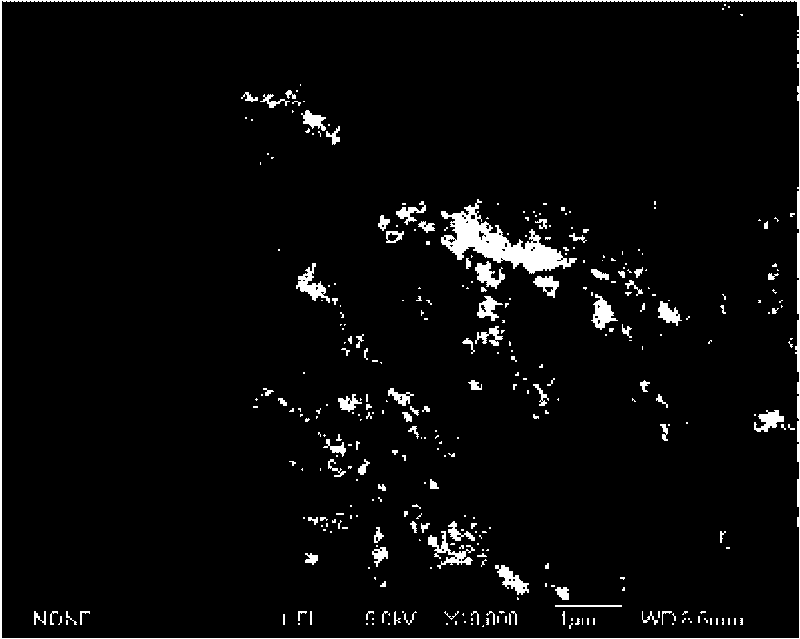
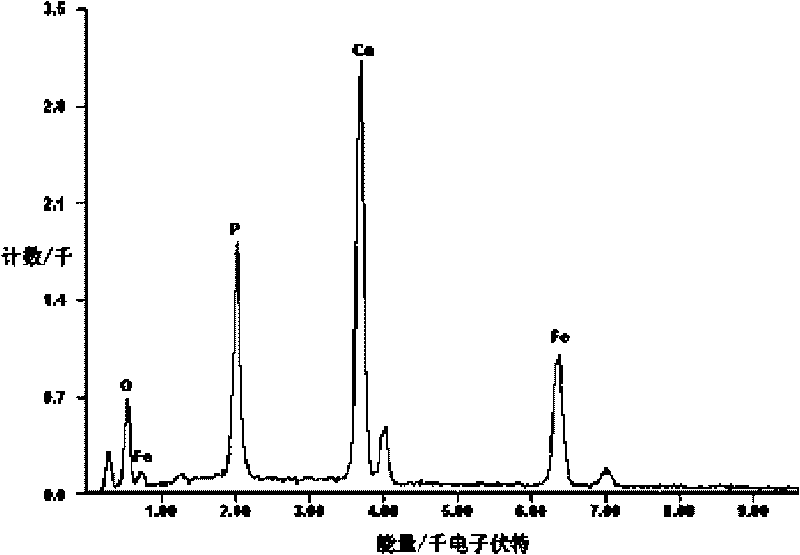
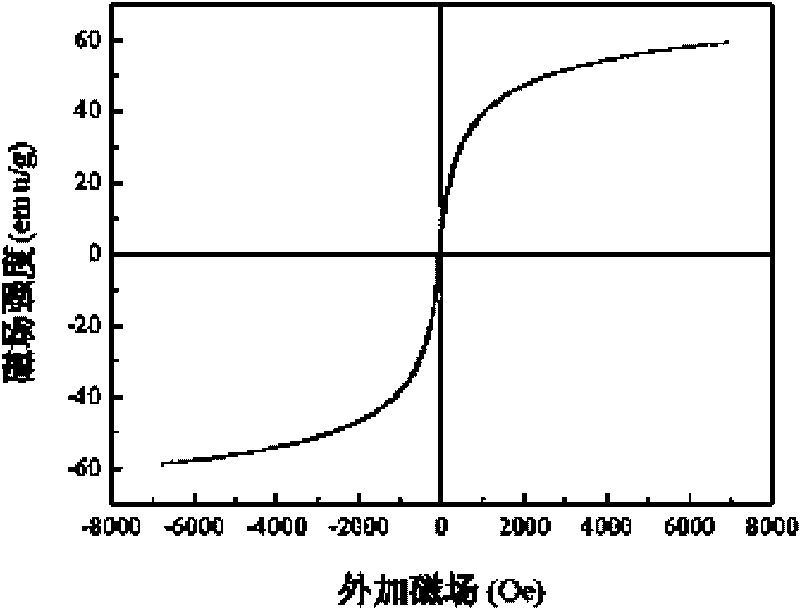
[0016] A magnetic nano-hydroxyapatite adsorbent, the adsorbent is based on nano-hydroxyapatite, and magnetic powder Fe is uniformly dispersed in the matrix 2 o 3 , the molecular formula of nano-hydroxyapatite is Ca 10 (PO 4 ) 6 (OH) 2 , nano-hydroxyapatite and magnetic powder Fe 2 o 3 The mass ratio of is about 1.36, that is, the magnetic powder Fe 2 o 3 The mass fraction in the adsorbent is about 42.4%.
[0017] The preparation method of the above-mentioned magnetic nano-hydroxyapatite adsorbent in this embodiment is as follows:
[0018] At room temperature and under nitrogen atmosphere, 1.85mmoL of FeCl 2 4H 2 O and 3.7 mmoL FeCl 3 ·6H 2 O was dissolved in 30mL of deoxygenated water, then 10mL of 25% ammonia solution was added to the solution and mechanically stirred for 15min, and a precipitate was obtained after stirring evenly, and then 50mL of Ca(NO 3 ) 2 (containing solute 33.67mmol) and 50mL of (NH 4 ) 2 HPO 4 (containing 20mmol of solute) solution was...
Embodiment 2
[0021] The magnetic nano-hydroxyapatite adsorbent of the present invention prepared in Example 1 was added to each 20mL Cd-containing 2+ , Zn 2+ In the wastewater (the initial concentration of heavy metal ions in each wastewater is shown in Table 1), the amount of the adsorbent in each wastewater is 0.1g / L, adjust the pH value to 5.0 ± 0.1, and use a water bath to keep the temperature at room temperature Oscillating adsorption with an oscillator, after 24 hours, use a magnet to separate the adsorbent from the above wastewater, and use flame atomic absorption spectrophotometry to measure the unadsorbed Cd in the wastewater 2+ , Zn2+ The results are shown in Table 1 below.
[0022] Table 1: Contents of heavy metal ions in wastewater before and after treatment
[0023] heavy metal ions in wastewater
Embodiment 3
[0025] The magnetic nano-hydroxyapatite adsorbent of the present invention that is made in embodiment 1 is added to 20mL containing Cd 2+ and Zn 2+ In the waste water of two kinds of heavy metal ions (the initial concentration of each heavy metal ion in the waste water is shown in Table 2), the consumption of adsorbent is 0.1g / L, adjusts pH value to 5.0 ± 0.1, utilizes water bath constant temperature shaker to carry out at room temperature Oscillating adsorption, after 24 hours, use a magnet to separate the adsorbent from the above wastewater, and use flame atomic absorption spectrophotometry to measure the unadsorbed Cd in the wastewater 2+ , Zn 2+ content. Table 2 lists the use of magnetic nano-hydroxyapatite adsorbent to simultaneously treat Cd-containing 2+ and Zn 2+ The removal effect of wastewater.
[0026] Table 2: Contents of heavy metal ions in wastewater before and after treatment
[0027] heavy metal ions in wastewater
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com