Isosorbide mononitrate sodium chloride injection
A technology of isosorbide dinitrate and sodium chloride injection, applied in the directions of active ingredients of heterocyclic compounds, medical preparations of non-active ingredients, cardiovascular system diseases, etc. and other problems to achieve the effect of ensuring product safety, avoiding secondary pollution, and simple disposal of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
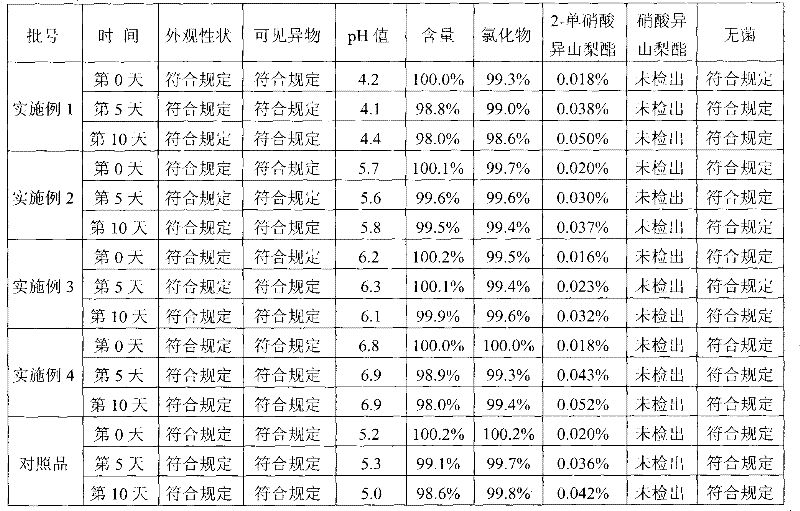
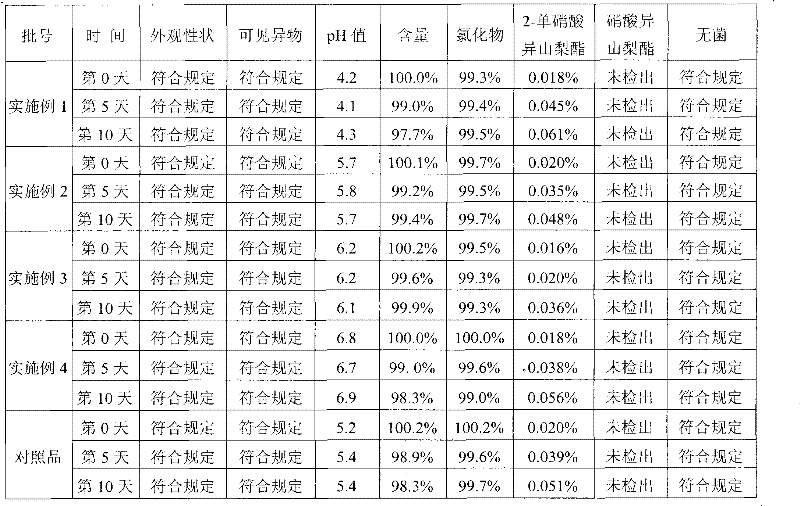
Image
Examples
Embodiment 1
[0027] Isosorbide mononitrate 10mg
[0028] Sodium chloride 450mg
[0029] 0.05mol / L sodium hydroxide solution appropriate amount
[0030] Add water for injection to 100ml
[0031] Preparation process: First prepare a concentrated solution of sodium chloride and filter, add isosorbide mononitrate and stir to dissolve, add water for injection to the full amount, adjust the pH value of the drug solution to 4.3 with an appropriate amount of 0.05mol / L sodium hydroxide solution, and fill and kill Bacteria, that is.
Embodiment 2
[0033] Isosorbide mononitrate 20mg
[0034] Sodium chloride 900mg
[0035] 0.05mol / L sodium hydroxide solution appropriate amount
[0036] Add water for injection to 100ml
[0037] Preparation process: First prepare a concentrated solution of sodium chloride and filter, add isosorbide mononitrate and stir to dissolve, add water for injection to the full amount, adjust the pH value of the drug solution to 5.7 with an appropriate amount of 0.05mol / L sodium hydroxide solution, and fill and kill Bacteria, that is.
Embodiment 3
[0039] Isosorbide mononitrate 20mg
[0040] Sodium chloride 900mg
[0041] 0.1mol / L sodium hydroxide solution appropriate amount
[0042] Add water for injection to 100ml
[0043] Preparation process: firstly prepare a concentrated solution of sodium chloride and filter, add isosorbide mononitrate and stir to dissolve, add water for injection to the full amount, adjust the pH value of the drug solution to 6.2 with an appropriate amount of 0.1mol / L sodium hydroxide solution, and fill and kill Bacteria, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com