Amphiphilic waterborne segmented copolymer medicament carrier with pH responsiveness and biodegradability and preparation thereof
A block copolymer and degradability technology, applied in the field of dual hydrophilic block copolymer drug carrier and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
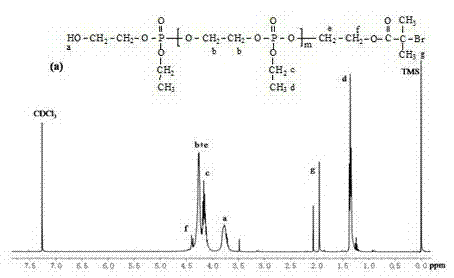
[0074] Embodiment one: prepare macromolecule ATRP initiator PEEP 32 The specific synthesis method of -Br:
[0075] (1) After drying the branched round-bottomed flask with a stirring bar in an oven at 120°C for at least 24 hours, plug it with a glass stopper, connect it to an oil pump through a latex tube, and pump it to room temperature, then introduce high-purity argon gas, and pump it again. Vacuum, so repeated three times.
[0076] (2) Inject a certain amount of initiator bromoisobutyrate-2-hydroxyethyl (HEBI) (0.14g, 0.66mmol) sequentially from the branch pipe with a dry syringe, 2-ethoxy-2-oxygen-1 , 3,2-dioxaphospholane (EEP) monomer (4g, 26mmol) and Sn(Oct) 2 (EEP monomer: Sn(Oct) 2 =1:0.002, mass ratio), anhydrous tetrahydrofuran (15 mL) was used as solvent. After the reaction flask was filled with argon, the reaction was stirred in an oil bath at 40° C. for 1 h.
[0077] (3) After the reaction, most of the solvent tetrahydrofuran was removed by rotary evaporation...
Embodiment 2
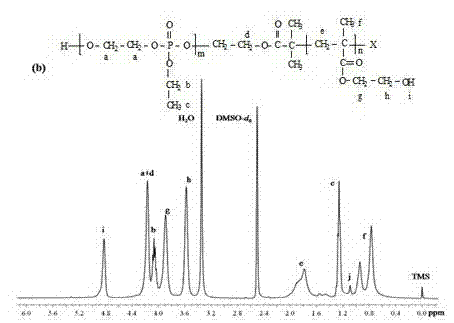
[0080] Embodiment two: diblock copolymer PEEP 32 -b-PHEMA 128 The specific preparation method:
[0081] (a) After treating the round-bottomed flask with branch tubes according to the above method, fill it with argon, quickly weigh and add PEEP 32 -Br (2.25g, 0.45mmol), cuprous bromide (0.06g, 0.45mmol) and bipyridine (0.14g, 0.45mmol) (the molar ratio of the three is 1:1:1), after repeated three times , inject 5 mL of solvent anhydrous methanol, stir until the mixture is completely dissolved and the solution turns brown.
[0082] (b) Move the round bottom flask to an oil bath, then add monomer hydroxyethyl methacrylate (HEMA) (8.7 g, 67.5 mmol) with a dry syringe, and use PEEP 32 The terminal bromine in the -Br homopolymer acts as an initiator to initiate the ATRP reaction, and the reaction is stirred in an oil bath at 30°C for 6h.
[0083] (c) After the reaction is over, contact with air to terminate the reaction, remove most of the solvent by rotary evaporation of the po...
Embodiment 3
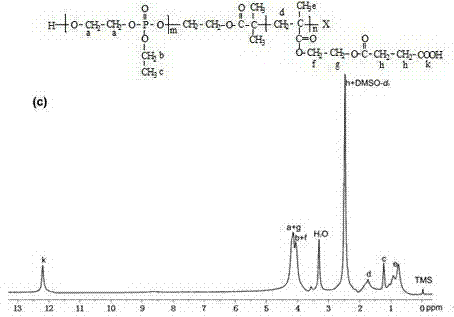
[0084] Example Three: pH Responsive Double Hydrophilic Block Copolymer PEEP 32 -b-PSEMA 128 The specific synthesis method:
[0085] (1) After the branch tube round bottom flask was treated according to the above method, it was filled with argon, and the above two-block polymer PEEP dissolved in 10mL of anhydrous pyridine was quickly added. 32 -b-PHEMA 128 (3g, the molar number of hydroxyl (HO-) is 16.64mmol), add succinic anhydride under magnetic stirring (3.328g, 33.28mmol) (the molar ratio of hydroxyl to succinic anhydride is 1:2), and reacted at room temperature for 48h.
[0086] (2) After the reaction is over, the reaction solution is precipitated in 150 mL of ice anhydrous ether to remove solvent and unreacted monomers. After repeated precipitation for 3 times, the product obtained after the precipitation is dissolved in deionized water and then dialyzed (dialysis Bag interception 3500g mol -1 ) for 2 to 3 days, and finally the dialysis solution was freeze-dried to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com