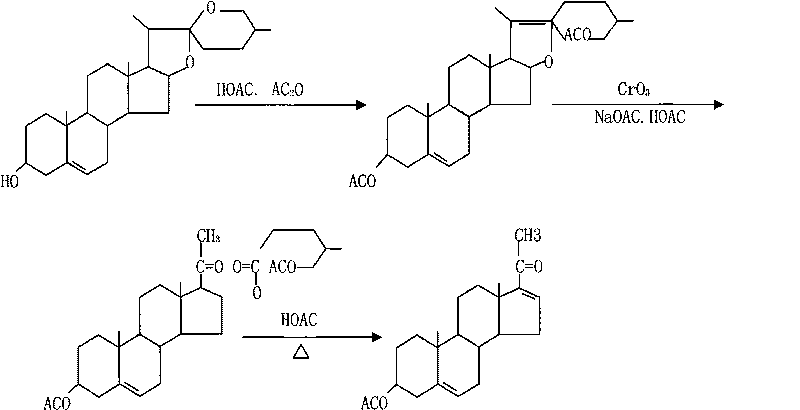
Method for preparing 16-dehydropregnenolone acetate by multistage filtration and recrystallization
A technology of dienol ketone acetate and recrystallization, applied in the direction of steroids, organic chemistry, etc., can solve the problem of increasing the difficulty of post-processing, unstable batch reactions, reducing product quality and product yield, and inconsistency in post-processing process changes. major problems, to achieve the effect of reducing difficulty, improving product quality and product yield, and reducing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Throw 160kg of diosgenin, add 88kg of glacial acetic acid, 252kg of acetic anhydride, and 96kg of mother liquor. When the internal pressure reaches 0.42MP by electric heating in an open-loop tank at 194°C, turn off the power and open the loop for 50 minutes. Finally, the internal temperature reaches 208 -210°C, pressure 0.57MPa ring opening is completed. Press it into the oxidation hydrolysis tank prepared with 90kg base acid, and start to lower the temperature. When the temperature in the tank drops to 10--12°C, close the brine, and immediately add the oxidant to the oxidation tank to carry out the oxidation reaction. When the temperature rose to 98°C, the reaction was timed for 25 minutes, and then steam was introduced into the jacket to heat up the reaction, and the acetic acid was recovered by distillation under normal pressure for 50 minutes, and then the glacial acetic acid was recovered under reduced pressure for 30 minutes. After the reaction is completed, the f...
Embodiment 2
[0026] The method for producing monoenolone acetate by multi-stage filtration and recrystallization, throws 160kg of sisal saponin, adds 88kg of glacial acetic acid, 252kg of acetic anhydride, and 96kg of mother liquor, and heats up in an open-loop tank at 194°C with an internal pressure of 0.42MP. a, power off timing. Open the ring for 50 minutes, and finally the internal temperature reaches 208-210°C, and the pressure is 0.57MPa to complete the ring opening. Press it into the oxidation hydrolysis tank prepared with 90kg base acid, and start to lower the temperature. When the temperature in the tank drops to 10--12°C, close the brine, and immediately add the oxidant to the oxidation tank to carry out the oxidation reaction. When the temperature rose to 98°C, the reaction was timed for 25 minutes, and then steam was introduced into the jacket to heat up the reaction, and the acetic acid was recovered by distillation under normal pressure for 50 minutes, and then the glacial ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com