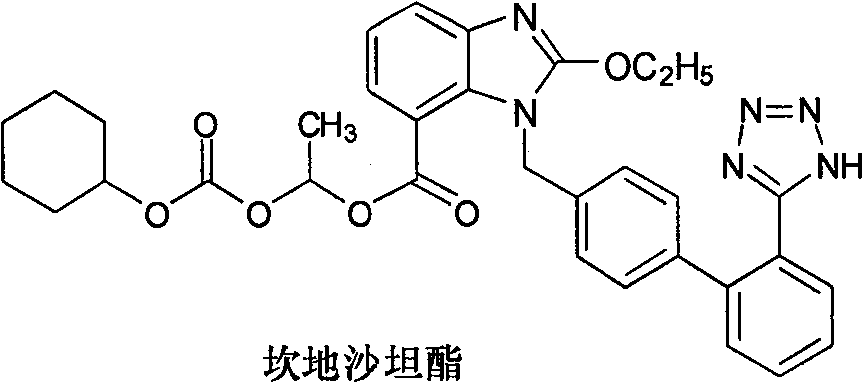
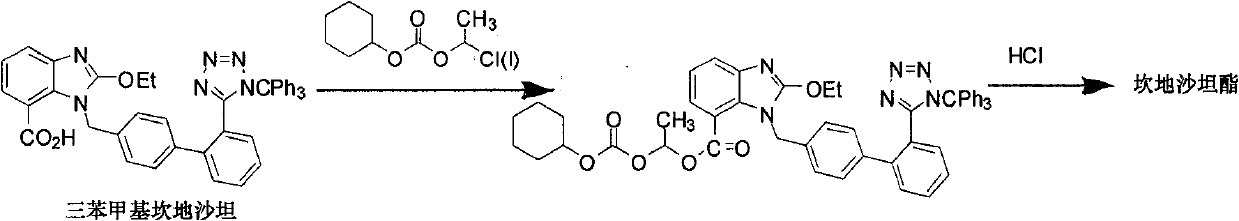
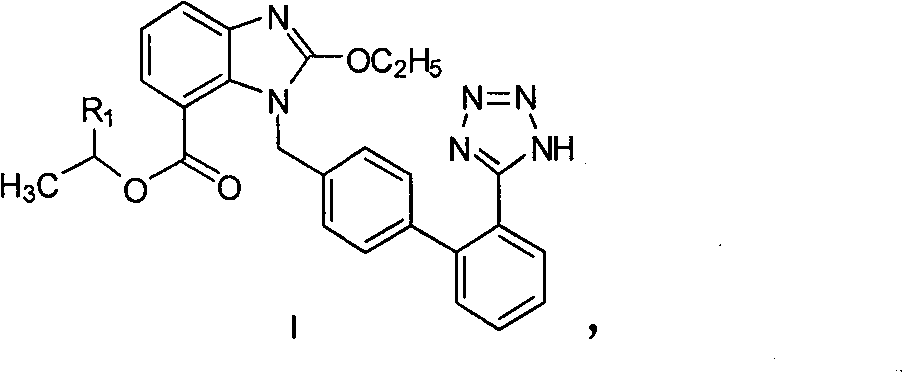
A preparation method of candesartan cilexetil and intermediates thereof
A compound and reaction technology, applied in the field of preparation of candesartan cilexetil, can solve the problems of inconvenient transportation and storage, poor stability, and difficulty in large-scale industrial production of candesartan cilexetil, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of cyclohexyl carbonate monoester
[0046] Ethyl chloroformate (7.5g, 0.069mol) was dissolved in dichloromethane (20ml), cyclohexanol (3.8g, 0.038mol) was added successively, and pyridine (1ml) was stirred at room temperature for about 20min. TLC detected no raw material, washed with water (10ml×2), dried over anhydrous sodium sulfate, and concentrated to obtain cyclohexylethyl carbonate (4.734g, 72.6%), a pale yellow oily liquid. MS (ESI) m / z: 173.8 (M+1), 172.8 (base peak), 80.1, 91.0, 128.1.
[0047]Dissolve cyclohexyl ethyl carbonate (4.248g, 0.025mol) in a mixed solution of ethanol and water (15ml, V (ethanol:water)=95:5), add sodium hydroxide (1.189g, 0.030mol) and stir at room temperature About 30min. TLC detects that there is no raw material, concentrate, add water (20ml), add dropwise hydrochloric acid to adjust the pH value of the solution to about 3, then extract with dichloromethane (10ml×2), dry over anhydrous sodium sulfate,...
Embodiment 2
[0048] Example 2: 1-chloroethyl-2-ethoxy-3-((2'-(1-trityl-1H-tetrazol-5-yl)biphenyl-4-yl)methanol Base)-3H-benzimidazole-4-ester (compound shown in formula II, R 1 =Cl) preparation
[0049] Mix trityl candesartan (212mg, 0.310mmol), 1,1-chlorobromoethane (437mg, 3.10mmol), potassium carbonate (100mg, 0.620mmol) and dry DMF (4ml), 20°C- The reaction is complete under the condition of 60° C. (specifically, 50° C. is selected to react for 24 hours). After cooling, pour into ice water (15ml), stir for half an hour, extract with ethyl acetate (10ml×3), wash the organic phase with water (10ml×2), wash with saturated brine (10ml), and dry over anhydrous sodium sulfate. Concentrate and recrystallize from acetone to give 1-chloroethyl-2-ethoxy-3-((2′-(1-trityl-1H-tetrazol-5-yl)biphenyl as a white solid Base-4-yl)methyl)-3H-benzimidazole-4-ester (compound shown in formula II, R 1 =Cl), yield 50%. MS (ESI) m / z: 745 (M+1).
Embodiment 3
[0050] Example 3: 1-chloroethyl-2-ethoxy-3-((2'-(1-trityl-1H-tetrazol-5-yl)biphenyl-4-yl)methanol Base)-3H-benzimidazole-4-ester (compound shown in formula II, R 1 =Cl) preparation
[0051] The 1,1-chlorobromoethane in Example 2 was replaced by 1,1-dichloroethane (294mg, 3.10mmol) to obtain 1-chloroethyl-2-ethoxy-3-( (2'-(1-trityl-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-3H-benzimidazole-4-ester (compound shown in formula II, R 1 =Cl), yield 35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com