Method for purifying copper electrolyte by using chemical reduction method
A copper electrolyte, electrolyte technology, applied in the direction of optics, improvement of process efficiency, photographic technology, etc., can solve the problems of large amount of precipitant, complicated operation, high tank voltage, etc., achieve good impurity removal effect, simple process, and tank The effect of high voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0007] In 1000mL decopper electrolyte, the composition of the decopper electrolyte is shown in Table 1, add 50mL hydrazine hydrate under stirring, when the reaction temperature is 42°C, filter after 60min, the removal rates of copper, arsenic, antimony and bismuth are respectively It is 97.04%, 5.28%, 37.92%, 94.40%. After filtration, the electrolyte returns to the electrolytic cell.
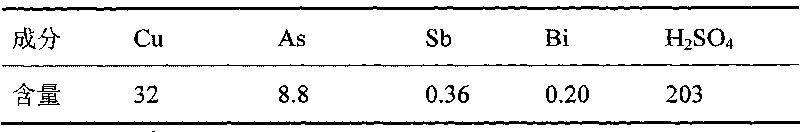
[0008] Table 1 Composition of decopper electrolyte g / L -1
[0009]
Embodiment 2
[0011] In 1000mL decopper electrolyte, the composition of decopper electrolyte is shown in Table 1, add 50mL hydrazine hydrate and 50mL concentrated hydrochloric acid (AR) under stirring, when the reaction temperature is 42°C, filter after 60min, copper, arsenic, The removal rates of antimony and bismuth are 98.77%, 19.04%, 21.76%, and 47%, respectively, and the electrolyte is returned to the electrolytic cell after filtration.
Embodiment 3
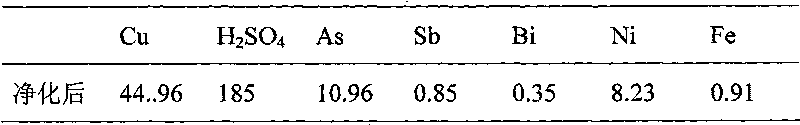
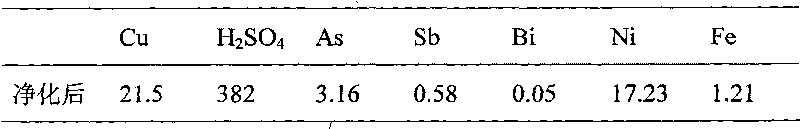
[0013] In 2000mL copper electrolyte, the composition of copper electrolyte is shown in Table 2. Sulfur dioxide is introduced at a flow rate of 600mL / min. After reduction to 4h at a reaction temperature of 65°C, the composition of copper electrolyte is evaporated by heating, crystallized by cooling and filtered. As shown in Table 3, the filtered electrolyte was returned to the electrolyzer.
[0014] Table 2 Composition of copper electrolyte / g L -1
[0015]
[0016] Table 3 Composition of copper electrolyte after purification by sulfur dioxide reduction, evaporation and crystallization / g L -1
[0017]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com