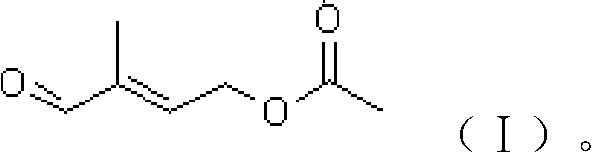
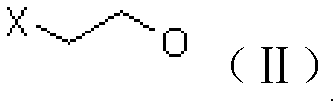Preparation method of 4-acetoxyl-2-methyl-2-butylenoic aldehyde
A technology of acetoxy and crotonaldehyde, applied in the field of preparation of 4-acetoxy-2-methyl-2-butenal, which can solve the problem of high equipment requirements and achieve good reaction selectivity and high yield , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
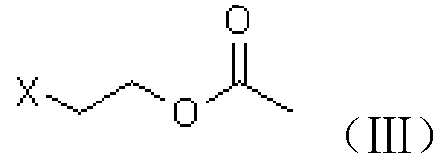
[0039] A. Preparation of chloroester (III)
[0040] Add 61g of acetic anhydride to a 250ml four-neck flask, add 3g of ion exchange resin loaded with periodic acid at room temperature, stir for about 15min, raise the temperature to 50°C, start adding 40g of 2-chloroethanol dropwise, finish the dripping in about 30min, and keep warm for the reaction 5h. After GC analysis, no 2-chloroethanol remains, and the temperature is lowered to about 10°C to filter out the catalyst-loaded periodic acid ion exchange resin, add 100ml of dichloromethane to the filtrate and dropwise add 10% sodium hydroxide solution to adjust the pH of the aqueous phase 6-7, the organic phase was separated, and the aqueous phase was extracted with dichloromethane and incorporated into the organic phase. The organic phase was washed once with 150 ml of saturated brine, and then dichloromethane was recovered under reduced pressure to obtain 60 g of chloroester (III), which was analyzed by GC with a content of 92...
Embodiment 2
[0049] A. Preparation of bromoester (III)
[0050] Add 61g of acetic anhydride to a 250ml four-neck flask, add 3g of ion exchange resin loaded with periodic acid at room temperature, stir for about 15min, heat up to 50°C, start to add 62g of 2-bromoethanol dropwise, finish dropping in about 30min, and keep warm for the reaction 5h. After GC analysis, there is no 2-bromoethanol residue, and the temperature is lowered to about 10°C to filter out the catalyst-loaded periodate ion exchange resin, add 100ml of dichloromethane to the filtrate and add 10% sodium hydroxide solution dropwise to adjust the pH of the water phase 6-7, the organic phase was separated, and the aqueous phase was extracted with dichloromethane and incorporated into the organic phase. The organic phase was washed once with 150 ml of saturated brine, and then dichloromethane was recovered under reduced pressure to obtain 82.5 g of bromoester (III). According to GC analysis, the content was 91.8%, and the yield...
Embodiment 3
[0059] A. prepare chloroester (III) with the same method of embodiment 1
[0060] B. Preparation of Phosphonate (IV)
[0061]Add 75g of triethyl phosphite into a 250ml four-neck flask, raise the temperature to 140°C, start to add 50g of chloroester (III) (content: 92.5%) dropwise, and finish dropping in about 45 minutes. temperature rise
[0062] Take a sample after holding the reaction for 1 hour at 150°C, and analyze by GC that the residual chloroester is less than 1%. Cool down to 70°C, remove the first part by depressurizing with an oil pump, until the temperature rises to 100°C and no leakage occurs, and finally 85.6g of phosphonate is obtained , GC test content 92.7%, yield 93.8%.
[0063] C. Preparation of five-carbon aldehyde acetal (V)
[0064] Add phosphonate (IV) 48g (content 92.7%) and 100ml toluene in a 250ml three-necked flask, stir for 5min, add 24.5g (content 96%) 1,1-dimethoxyacetone, under normal temperature (20-30 ℃) Add 14g of sodium hydroxide, and keep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com