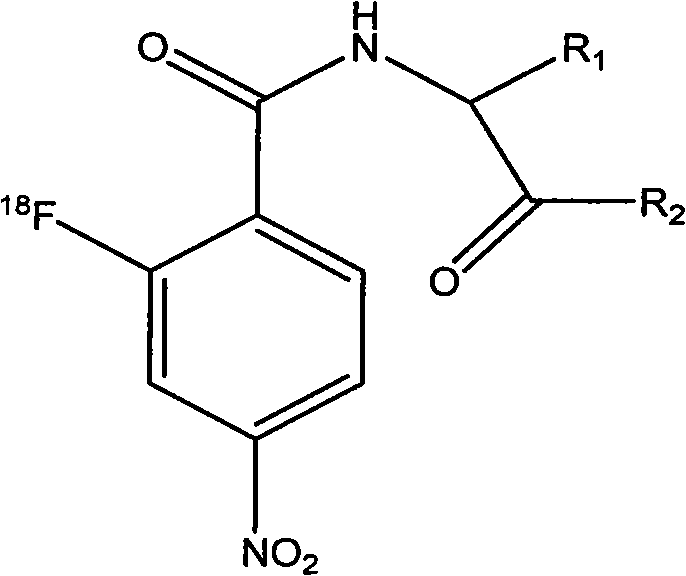
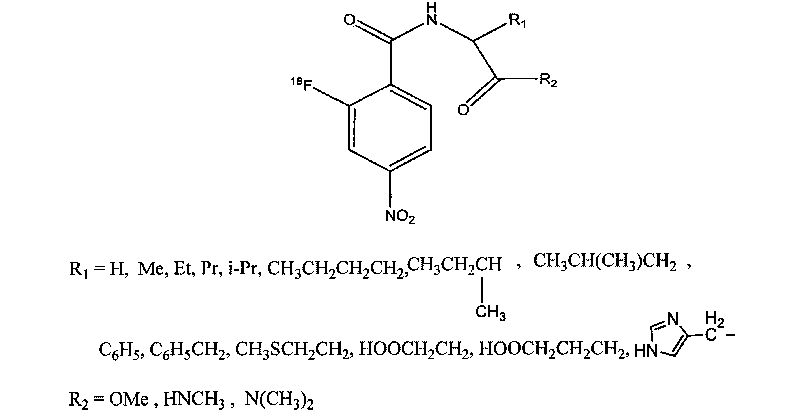
Novel 18F labeled p-nitro benzoyl amino acid derivatives, preparation method and application thereof in tumor imaging
A technology of nitrobenzoyl amino acid and labeling method, which is applied in the field of novel 18F-labeled p-nitrobenzoyl amino acid derivatives, preparation and application in tumor imaging, achieving less radioactive loss, simple synthesis, and raw materials Inexpensive and easy to get effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0080] Prepared according to the following steps is R in formula A 1 The group is isopropyl, R 2 The compound whose radical is a methoxyl group includes a labeling precursor (R in formula B 1 The group is isopropyl, R 2 The synthesis of compounds whose group is methoxy) and the synthesis of precursor compounds 18 F marks two parts.
[0081] 1) labeled precursor (R in formula B 1 The group is isopropyl, R 2 Synthesis of compounds whose group is methoxy)
[0082] 1.1 Synthesis of 2,4-dinitrobenzoic acid (formula J)
[0083] In a three-necked flask, add 5.5 grams (0.03 mol) of 2,4-dinitrotoluene to 30 mL of pyridine and 50 mL of water, heat, and maintain the temperature at 78-83 ° C, and add 32 g of potassium permanganate in batches ( 0.202mol), after adding in 6-8h, wash the potassium permanganate sticking to the inner wall of the condenser tube end with 10mL of water into the bottle, continue to react for 3h, until the potassium permanganate is completely faded, filter w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com