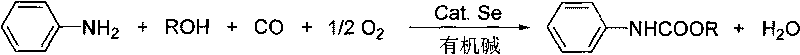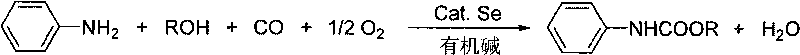Method for synthesizing phenyl carbamate
A kind of phenylcarbamate, one party's technology, applied in the field of synthesis of phenylcarbamate, can solve the problems of increased difficulty of separation and purification, increased reaction cost, low atom economy, etc., and achieves easy separation and purification of products, simple structure, The effect of short reaction routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In 100ml stainless steel autoclave, add aniline (5mmol), Se (0.25mmol), ethanol (50mmol), Et 3 N (10mmol), with CO and O 2 The mixed gas (molar ratio 4:1) was replaced three times and the pressure of the mixed gas was raised to 2.0MPa. After stirring and reacting this at 180° C. for 6 hours, the reaction was stopped. The kettle was opened to deflate, and the reaction mixture was left open and stirred at room temperature for another 0.5 hour to fully precipitate selenium. Then filter to recycle Se. The filtrate was concentrated, and the product was purified by column chromatography, and the eluent was petroleum ether: ethyl acetate (15:1-10:1). Concentrate and remove the eluent to obtain the target product N-phenylcarbamate with a yield of 85%. It can also be directly recrystallized in petroleum ether to obtain colorless needle-like crystals.
Embodiment 2
[0036] The alcohol is propanol, and the dosage is 50 mmol. Other experimental methods and conditions are the same as in Example 1. The product is N-phenylcarbamate propyl ester, and the actual yield is 87%.
Embodiment 3
[0038] The alcohol is isopropanol, and the consumption is 50 mmol. Other experimental methods and conditions are the same as in Example 1. The product is isopropyl N-phenylcarbamate, and the actual yield is 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com