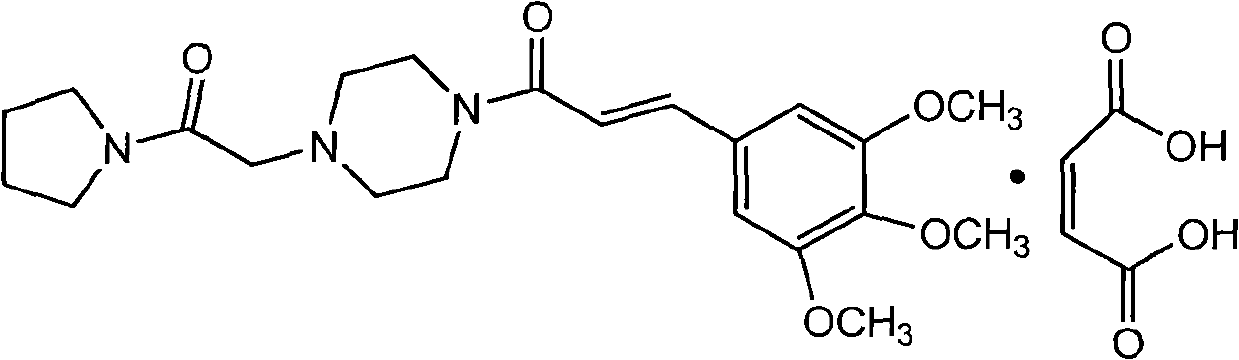
Preparation method of cinepazide maleate
A technology of cinepazide maleate and cinnamyl piperazine is applied in a preparation field of cinepazide maleate, which can solve the problems of unstable amide, poor stability and high risk, and achieve simplified production conditions and process, stable crystal form, high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
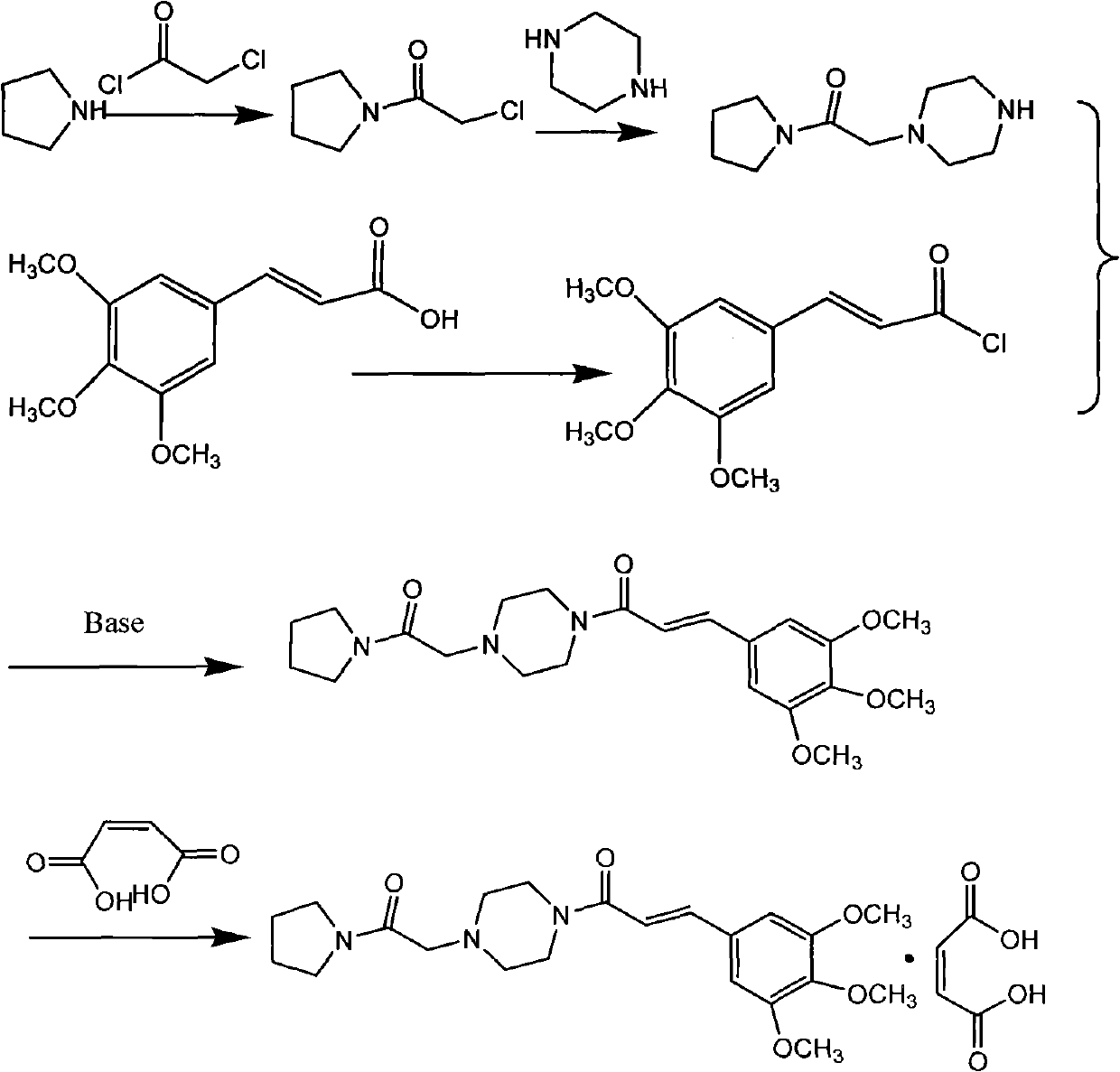
Embodiment 1
[0031] 1. Solvent-free synthesis of chloroacetylpyrrolidine:
[0032] Add 142 grams (2 mol) of tetrahydropyrrole dropwise to 113 grams (1 mol) of chloroacetyl chloride solution under stirring, control the rate of addition and the temperature during the addition (controlled within the range of -10 to 10°C), and the addition is completed Then continue to stir and naturally warm up to room temperature (25°C) and stir for 2 hours; add a 1:3 ethyl acetate / petroleum ether mixed solution with a weight ratio of 1:3 to the reaction system to precipitate tetrahydropyrrole hydrochloride, and filter and recover tetrahydropyrrole Hydrochloride, tetrahydropyrrole was obtained after alkalization, repeated use, the filtrate was evaporated to dryness, and solidified at room temperature to obtain 121 g of chloroacetylpyrrolidine. Melting point 42-45 ° C
[0033] 2. Synthesis of (E)-3,4,5-trimethoxycinnamoylpiperazine:
[0034] 120 g (0.5 mol) of (E)-3,4,5-trimethoxycinnamic acid, 101 g (10 mol...
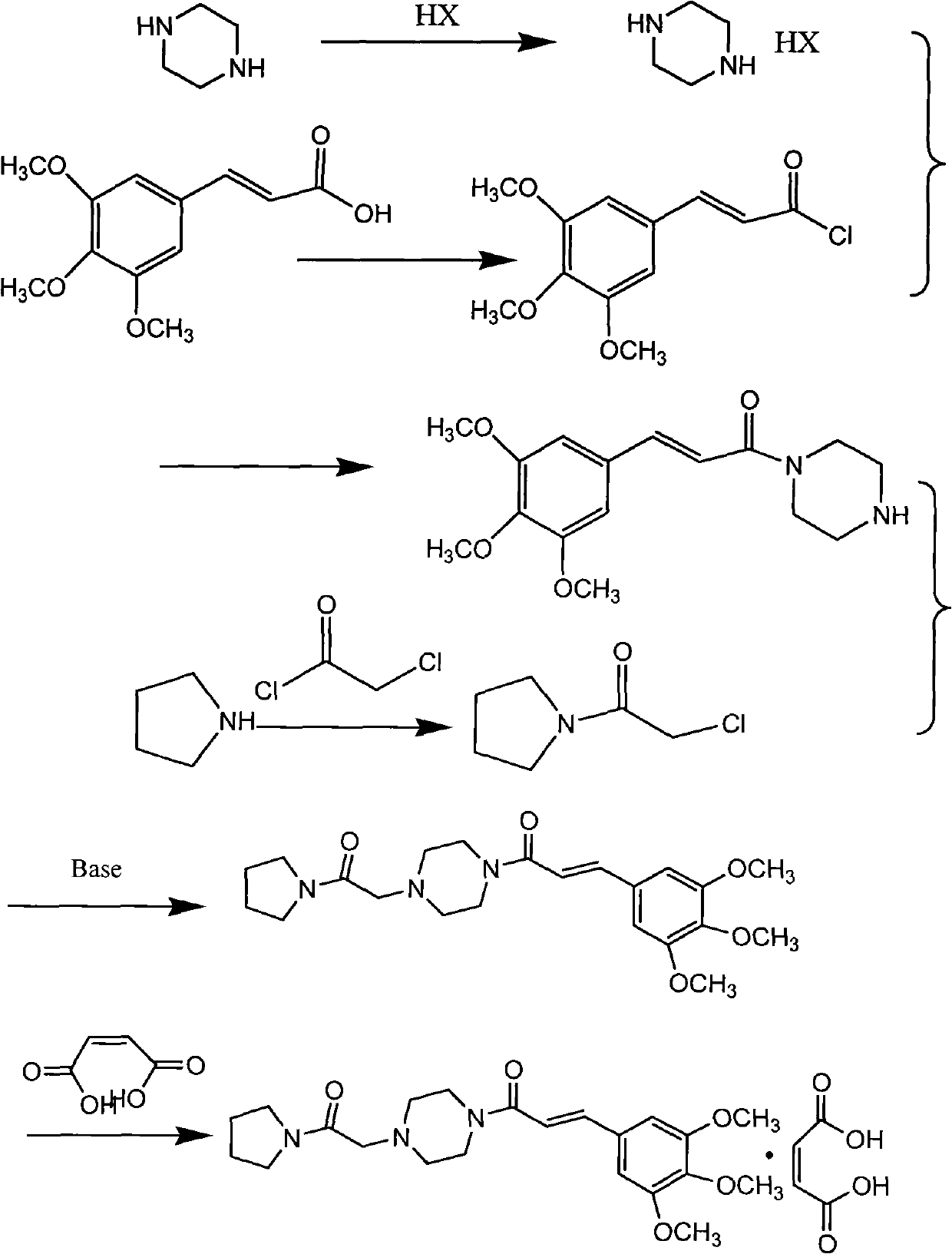
Embodiment 2
[0038] 1. Solvent-free synthesis of chloroacetylpyrrolidine:
[0039] Add 142 grams (2 mol) of tetrahydropyrrole dropwise to 113 grams (1 mol) of chloroacetyl chloride solution under stirring, control the rate of addition and the temperature during the addition (controlled within the range of -10 to 10°C), and the addition is completed Then continue to stir and naturally warm up to room temperature (25°C) and stir for 2 hours; add a 1:3 ethyl acetate / petroleum ether mixed solution with a weight ratio of 1:3 to the reaction system to precipitate tetrahydropyrrole hydrochloride, and filter and recover tetrahydropyrrole Hydrochloride, tetrahydropyrrole was obtained after alkalization, repeated use, the filtrate was evaporated to dryness, and solidified at room temperature to obtain 121 g of chloroacetylpyrrolidine. Melting point 42-45 ° C
[0040] 2. Synthesis of (E)-3,4,5-trimethoxycinnamoylpiperazine:
[0041] 120 g (0.5 mol) of (E)-3,4,5-trimethoxycinnamic acid, 101 g (10 mol...
Embodiment 3
[0045] 1. Solvent-free synthesis of chloroacetylpyrrolidine:
[0046] Add 142 grams (2 mol) of tetrahydropyrrole dropwise to 113 grams (1 mol) of chloroacetyl chloride solution under stirring, control the rate of addition and the temperature during the addition (controlled within the range of -10 to 10°C), and the addition is completed Then continue to stir and naturally warm up to room temperature (25°C) and stir for 2 hours; add a 1:3 ethyl acetate / petroleum ether mixed solution with a weight ratio of 1:3 to the reaction system to precipitate tetrahydropyrrole hydrochloride, and filter and recover tetrahydropyrrole Hydrochloride, tetrahydropyrrole was obtained after alkalization, repeated use, the filtrate was evaporated to dryness, and solidified at room temperature to obtain 121 g of chloroacetylpyrrolidine. Melting point 42-45 ° C
[0047] 2. Synthesis of (E)-3,4,5-trimethoxycinnamoylpiperazine:
[0048] 120 g (0.5 mol) of (E)-3,4,5-trimethoxycinnamic acid and 300 mL of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com