Plant pathogenic fungi fusarium moniliforme natural antagonist
A technology of Fusarium moniliformes and compounds, which can be applied in the directions of plant growth regulators, fungicides, fungi, etc., can solve the problems that do not involve Fusarium nidulans, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The separation of embodiment 1 Pseudomonas fumigatus (P.adusta) SN17
[0018] P.adusta SN17, which was isolated from Hainan Province, was deposited in the General Microorganism Center (CGMCC) of China Committee for Culture Collection of Microorganisms on September 26, 2008 (大学, Chaoyang District, Beijing, China). Tunlu Institute of Microbiology, Chinese Academy of Sciences, 100101), the preservation number is 2672.
[0019] The separation method adopts the conventional endophytic fungus separation method, and the specific steps are as follows: wash the healthy plant branches and leaves with water, dry them, soak them in 75% alcohol for disinfection for 60 seconds, soak them in 25% (1.3% available chlorine) sodium hypochlorite Disinfect for 5 minutes, then soak and disinfect with 75% alcohol for 30 seconds, then wash with sterile water for 3 times, cut the sterilized tissue into small pieces with a side length of about 5mm, place them on PDA medium, and place at 25°C to...
Embodiment 2
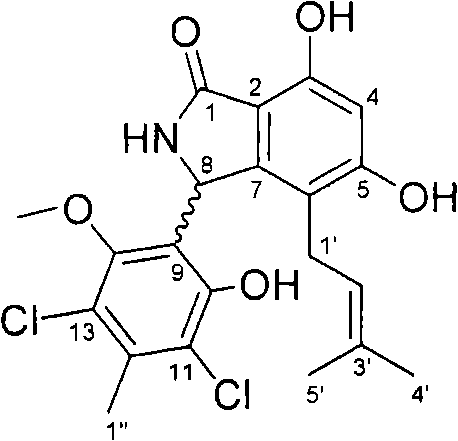
[0029] Example 2 Isolation and preparation of compound chlorinated discoidin A
[0030] Materials: Potatoes are commercially available, and other reagents used in the present invention are of analytical grade, which are sold by general biological reagent companies.
[0031] a. Activation of P.adusta SN17:
[0032] Prepare PDA medium: 200g of potatoes, 20g of glucose, 15g of agar, 1000mL of water, make a test tube slant, pick mycelium and inoculate it on the test tube slant, and incubate at 25°C for 10 days;
[0033] b. Solid fermentation culture of Discocystis fumigatus SN17:
[0034] Prepare solid medium, and its preparation process is: 150mL liquid medium (containing 6% dextrin, 2% maltose, 0.75% cottonseed meal, 0.7% peptone, 0.25% CaCO 3 , 0.25% MgSO 4 ·7H 2 O, 0.1% FeSO 4 ·7H 2 O, 0.001% ZnSO 4 , adjust the pH to 6.0) and 30g of vermiculite into a 500mL Erlenmeyer flask, prepare 10 parts, soak overnight, autoclave at 121°C for 30 minutes, and cool for later use;
...
Embodiment 3
[0040] Physicochemical analysis of embodiment 3 chlorinated discoidin A
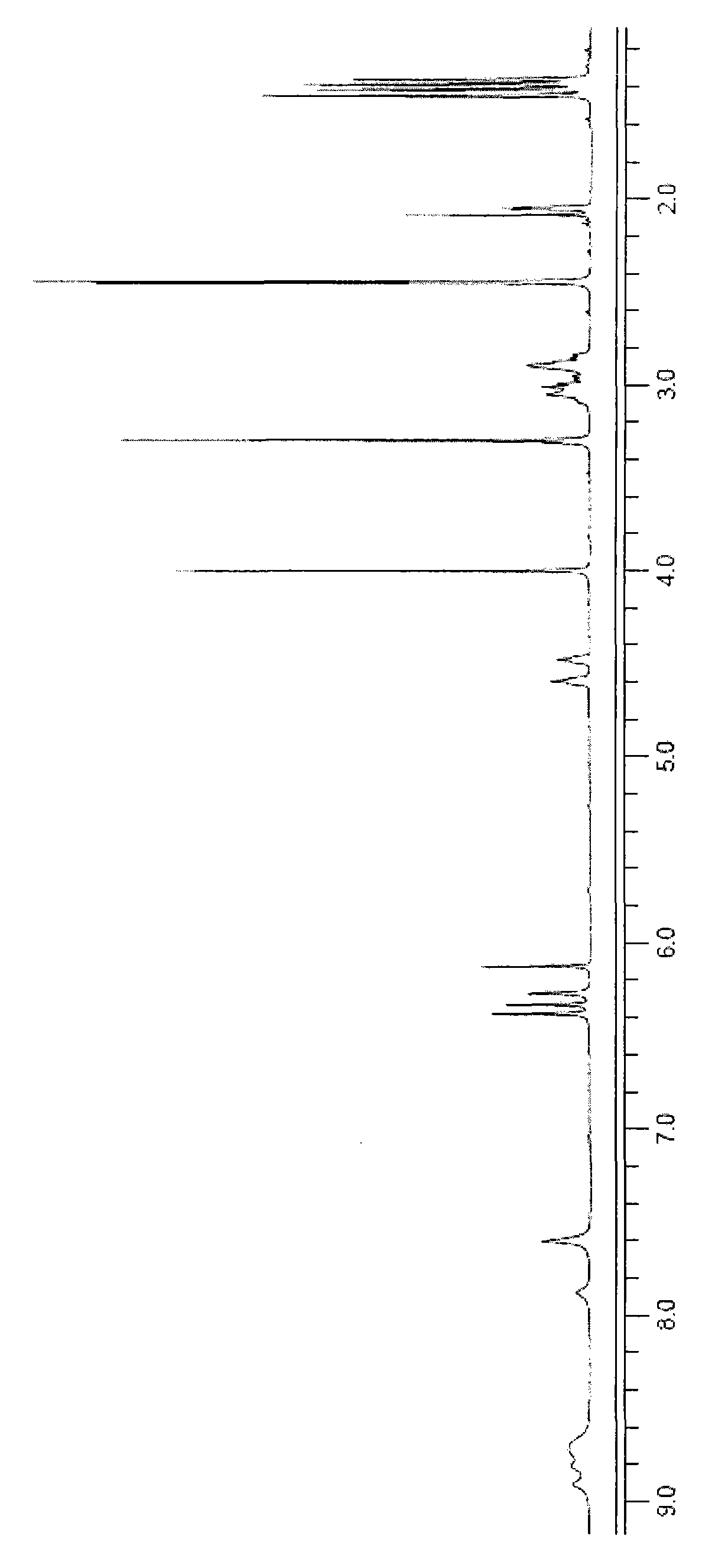
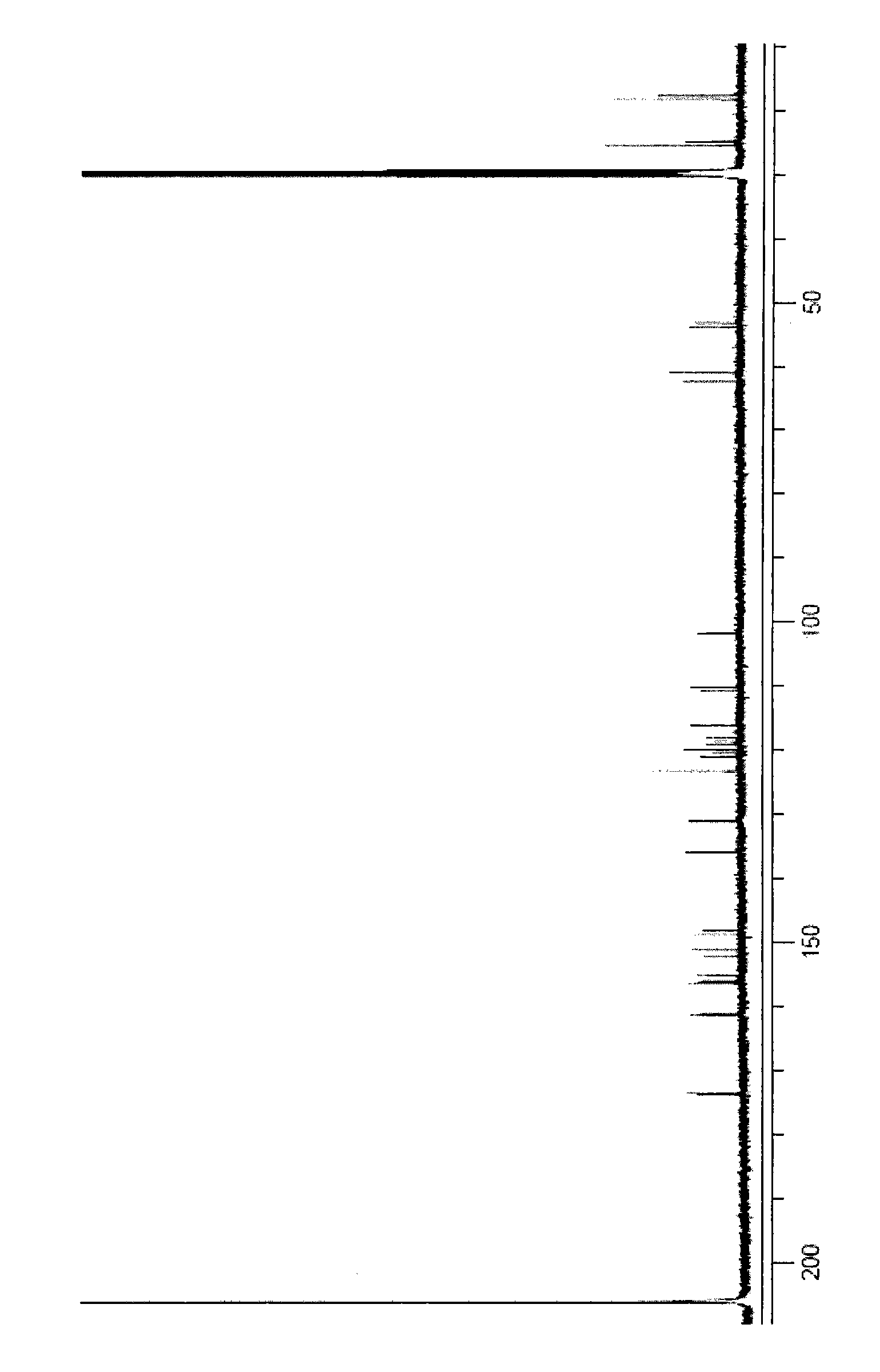
[0041] The chlorinated discoidin A obtained in step e of Example 2 has the following physical and chemical properties: the shape is a colorless flaky crystal, and the melting point is 205-207 ° C; molecular formula: C 21 h 21 Cl 2 NO 5 ; Molecular weight: 437 (according to ESI-MS); Specific rotation [α] D +5.0(c 0.4, CH 3 OH), ultraviolet UV (CH 3 OH)λ max =211 (ε100100), 253 (ε17600), 296 (ε12200) nm. The mass spectrum and infrared spectrum data of chlorinated discoidin A are shown in Table 1, and the hydrogen spectrum and carbon spectrum data are shown in Table 2. 1 H NMR spectrum see figure 1 , 13 C NMR spectrum see figure 2 .
[0042] Table 1. Physicochemical constants of chlorinated discoidin A
[0043]
[0044] Table 2. Hydrogen spectrum and carbon spectrum data of chlorinated discoidin A (deuterated acetone)
[0045]
[0046] s-singlet; d-doublet; t-triplet; m-multiplet
[004...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com