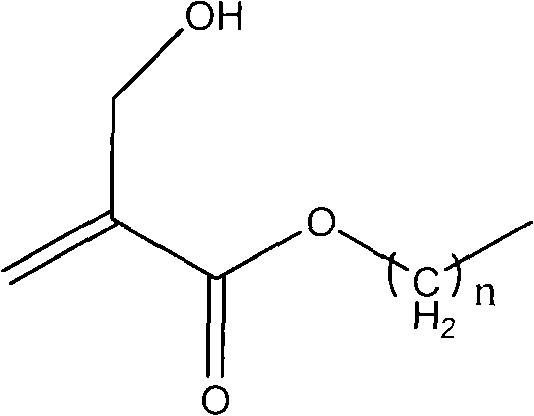
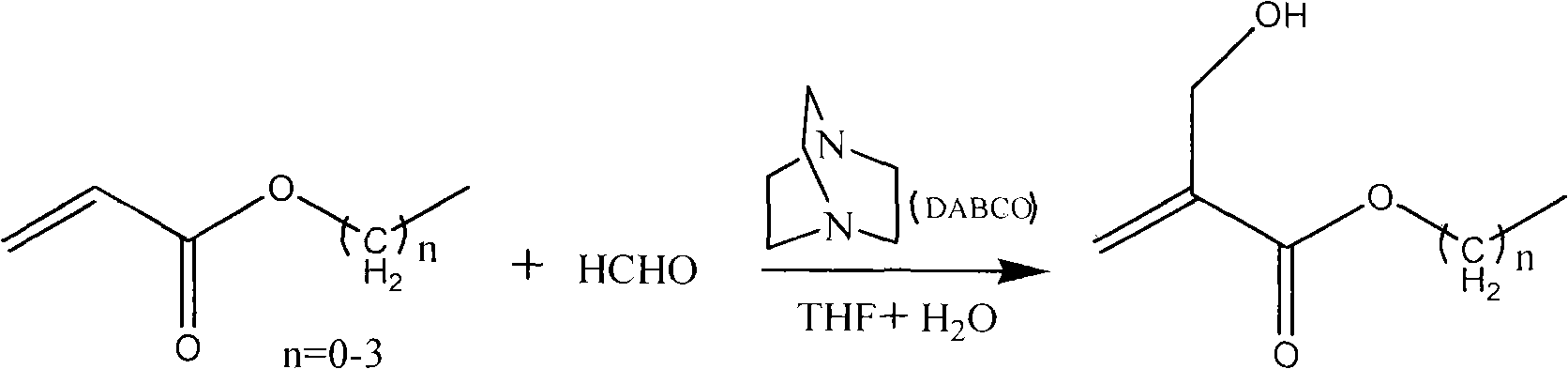
Method for synthesizing 2-hydroxymethyl acrylate compound
A technology of methylol acrylate and acrylate, applied in the field of synthesis of 2-hydroxymethylacrylate compounds, can solve problems such as slow reaction speed and low reaction yield, achieve easy operation and reduce acetal side reactions , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the synthesis of ethyl 2-hydroxymethacrylate
[0017] Add 33.4g of paraformaldehyde, 60g of water, and 1.7ml of phosphoric acid (1mol / L) into a 250ml reaction bottle, seal and stir to heat up to 95°C, react for 4 hours, and cool to room temperature for later use. Add 100g of ethyl acrylate, 16.7g of DABCO, 0.08g of p-methoxyphenol, and 100ml of THF into a 1000ml reaction bottle, stir at room temperature until completely dissolved, then add the prepared formaldehyde solution, and add 3ml of 30% sodium hydroxide solution at a uniform speed, Adjust the pH of the system to be alkaline. Stir for 4.5 hours for post-treatment: add 20 g of sodium chloride to saturate the aqueous phase, adjust the pH to 7 with hydrochloric acid, separate the liquids, add 20 g of anhydrous sodium sulfate to the organic phase, stir for 2 hours, and filter. After removing the solvent, the filtrate was distilled under reduced pressure, and the fraction at 120-122°C / 130Pa was received ...
Embodiment 2
[0018] Embodiment 2: the synthesis of 2-hydroxypropyl methacrylate
[0019] Add 45g of paraformaldehyde, 80g of water, and 2.3ml of phosphoric acid (1mol / L) into a 250ml reaction bottle, seal and stir to heat up to 80°C, react for 4 hours, and cool to room temperature for later use. Add 100g of propyl acrylate, 9.6g of DABCO, and 100ml of THF into a 1000ml reaction bottle, stir at 50°C until completely dissolved, add the prepared aqueous formaldehyde solution, add 4ml of 30% potassium hydroxide solution, adjust the pH of the system to be alkaline, and stir for 6 hours Post-treatment: add 20 g of sodium chloride to saturate the aqueous phase, adjust the pH value to 7 with hydrochloric acid, separate the liquids, add 20 g of anhydrous sodium sulfate to the organic phase, stir for 2 hours, and filter. After removing the solvent, the filtrate was distilled under reduced pressure to obtain 115 g of 2-hydroxypropyl methacrylate with a purity of 99.0% and a yield of 88.5%.
Embodiment 3
[0020] Embodiment 3: the synthesis of 2-methyl hydroxymethacrylate
[0021] Add 45g of paraformaldehyde, 80g of water, and 2.3ml of 1mol / L phosphoric acid into a 250ml reaction bottle, seal and stir to heat up to 95°C, react for 4 hours, and cool to room temperature for later use. Add 86g of methyl acrylate, 9.6g of DABCO, and 100ml of THF into a 1000ml reaction bottle, stir until completely dissolved at 80°C, add the prepared aqueous formaldehyde solution at 85°C, add 4ml of 30% sodium carbonate solution, and adjust the pH of the system to be alkaline. Stir for 8 hours for post-treatment: add 20 g of sodium chloride to saturate the aqueous phase, adjust the pH to 7 with hydrochloric acid, separate the liquids, add 20 g of anhydrous sodium sulfate to the organic phase, stir for 2 hours, and filter. After removing the solvent, the filtrate was distilled under reduced pressure, and the fraction received was 110-112° C. / 130 Pa to obtain 95 g of methyl 2-hydroxymethacrylate with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com