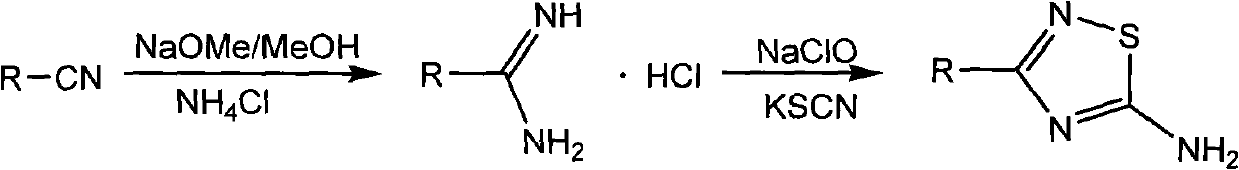5-amino-1,2,4-thiadiazole compound and preparation method thereof
A technology for thiadiazoles and compounds, which is applied in the field of organic chemical synthesis, can solve the problems of unsuitable industrial production, complicated preparation methods, increased cost and the like, and achieves the effects of avoiding corrosiveness and toxicity, simple reaction process and reducing pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
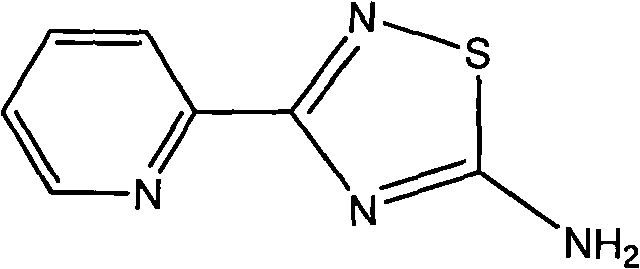
[0012] Example 1: Synthesis of 3-(2-pyridyl)-5-amino-1,2,4-thiadiazole
[0013] Weigh 52g (0.5mol) of 2-cyanopyridine in a 250mL round-bottomed flask, add 200mL of methanol to dissolve it, add 5.6g (0.052mol) of methanol solution of sodium methoxide with a mass percentage of 50%, and magnetically stir the reaction at room temperature 3h. After adding 27.5 g (0.515 mol) of ammonium chloride into the above system, the reaction was continued at room temperature for 48 h. The end point of the reaction was tracked by TLC. After the reaction was completed, the solvent was evaporated to dryness, and the remaining solid was washed with ether, filtered, and repeated three times. The filter cake was recrystallized with hot water (about 60 ° C) and ethanol in turn to obtain white needle-shaped crystals of 2-formamidinyl Pyridine hydrochloride 66.9g, yield 85%.
[0014] Weigh 59.0 g (0.375 mol) of 2-formamidinopyridine hydrochloride and place it in a 500 mL round-bottomed flask, add 250...
Embodiment 2
[0020] Example 2: Synthesis of 3-(3-pyridyl)-5-amino-1,2,4-thiadiazole
[0021] Weigh 52g (0.5mol) of 3-cyanopyridine in a 250mL round bottom flask, add 200mL of methanol to dissolve it, add 5.6g (0.052mol) of methanol solution of sodium methoxide with a mass percentage of 50%, and magnetically stir the reaction at room temperature 4h. After adding 27.5 g (0.515 mol) of ammonium chloride into the above system, the reaction was continued at room temperature for 48 h. The end point of the reaction was tracked by TLC. After the reaction was completed, the solvent was evaporated to dryness. The remaining solid was washed with ether, filtered, and repeated three times. Pyridine hydrochloride 60.5g, yield 76.8%.
[0022] Weigh 59.0 g (0.375 mol) of 3-formamidinopyridine hydrochloride and place it in a 500 mL round bottom flask, add 250 mL of water to dissolve it, and add 280 g (0.376 mol) of 10% sodium hypochlorite aqueous solution dropwise under ice-water bath conditions . Afte...
Embodiment 3
[0028] Example 3: Synthesis of 3-(4-pyridyl)-5-amino-1,2,4-thiadiazole
[0029] Weigh 52g (0.5mol) of 4-cyanopyridine in a 250mL round bottom flask, add 200mL of methanol to dissolve it, add 5.6g (0.052mol) of methanol solution of sodium methoxide with a mass percentage of 50%, and magnetically stir the reaction at room temperature 4h. After adding 27.5 g (0.515 mol) of ammonium chloride into the above system, the reaction was continued at room temperature for 48 h. The end point of the reaction was tracked by TLC. After the reaction was completed, the solvent was evaporated to dryness, and the remaining solid was washed with ether, filtered, and repeated three times. The filter cake was recrystallized with hot water (about 60 ° C) and ethanol in turn to obtain white needle-shaped crystals of 4-formamidino Pyridine hydrochloride 72.4g, yield 92%.
[0030] Weigh 59.0 g (0.375 mol) of 4-formamidinopyridine hydrochloride and place it in a 500 mL round bottom flask, add 250 mL o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com