Interpenetrating network ion exchange membrane based on polyurethane and preparation method thereof
An ion exchange membrane and interpenetrating network technology, which is applied in the field of polymer functional membrane materials, can solve the problems of difficult control of the composite process of membrane materials, difficulty in large-scale batch production, environmental pollution of sulfonating agents, etc., so as to reduce membrane proton exchange. The effect of resistance, strong electrochemical corrosion resistance, and simple film forming method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
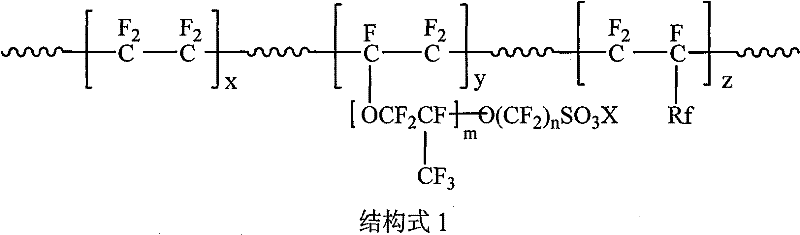
[0034] With 120g dry sulfonic acid resin (number average molecular weight 80,000, exchange capacity 1.25mmol / g, H + type) was dissolved in 880g N,N-dimethylformamide (DMF) under anhydrous conditions to obtain a sulfonic acid solution (casting solution), adding anhydrous toluene-2,4-diisocyanate 4.5g, anhydrous Trimethylolpropane (TMP) 3.5g (ratio of both hydroxyl-OH numbers and cyanate group-NCO numbers is 1:1), after fully dissolving and stirring evenly, under anhydrous conditions in a smooth and horizontal The surface of the glass is salivated, the solvent is evaporated at 75°C for 10 hours to form a film, and the ion exchange membrane is obtained after peeling off the glass, and fluorinated with fluorine gas to obtain an ion exchange membrane with an interpenetrating network structure with a film thickness of 50 microns.
Embodiment 2
[0036] With 45g dry sulfonic acid resin (number average molecular weight 150,000, exchange capacity 2.55mmol / g, Na + type) was dissolved in 880g N-methyl-2-pyrrolidone (NMP) under anhydrous conditions to obtain a sulfonic acid solution (casting solution), and anhydrous 4,4'-diphenylmethane diisocyanate (MDI) was added A total of 44g of anhydrous pentaerythritol (the ratio of the number of hydroxyl groups-OH to the number of cyanate groups-NCO is 1:0.8), fully dissolved and stirred evenly, under anhydrous conditions on the surface of a smooth and horizontal Hastelloy plate Salivate, heat up to 150°C to evaporate the solvent for 1 hour to form a film, and peel off from the glass to obtain an ion-exchange membrane with an interpenetrating network structure with a film thickness of 20 microns.
Embodiment 3
[0038] With 420g dry sulfonic acid resin (number average molecular weight 250,000, exchange capacity 1.05mmol / g, Na + type) was dissolved in 880g N,N-dimethylacetamide (DMAc) under anhydrous conditions to obtain a sulfonic acid solution (casting solution), adding anhydrous triphenylmethane triisocyanate (TTI), anhydrous A total of 210g of diethylene glycol acetal (the ratio of the number of hydroxyl groups - OH to the number of cyanate groups - NCO is 1: 1.5), after fully dissolving and stirring evenly, drool on a smooth and horizontal glass surface under anhydrous conditions , The solvent was evaporated at 100°C for 2 hours to form a film, and the ion exchange membrane was obtained after peeling off from the glass, and fluorinated with fluorine gas to obtain an ion exchange membrane with an interpenetrating network structure with a film thickness of 150 microns.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com