Listeria monocytogenes fluorescence quantitative PCR (Polymerase Chain Reaction) test kit and test method
A technology of mononucleosis and detection kits, which is applied in the determination/testing of microorganisms, biochemical equipment and methods, fluorescence/phosphorescence, etc., and can solve the problems of inaccurate quantification of false positives, difficulties in the preparation of monoclonal antibodies, cross-reactions, etc. problems, to achieve the effects of accurate quantification, high detection specificity, and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
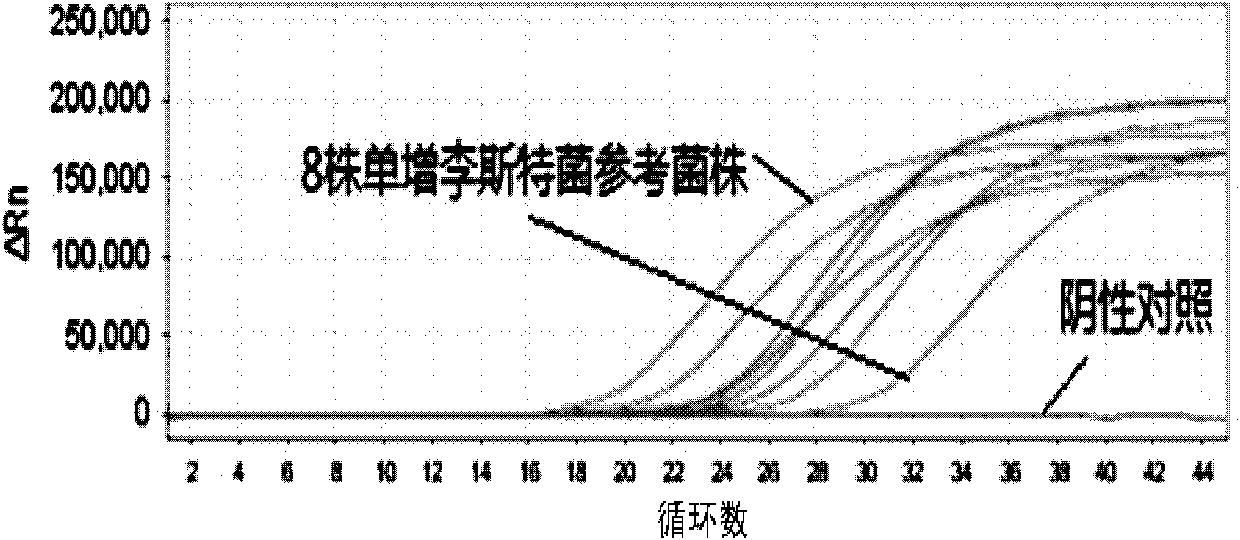
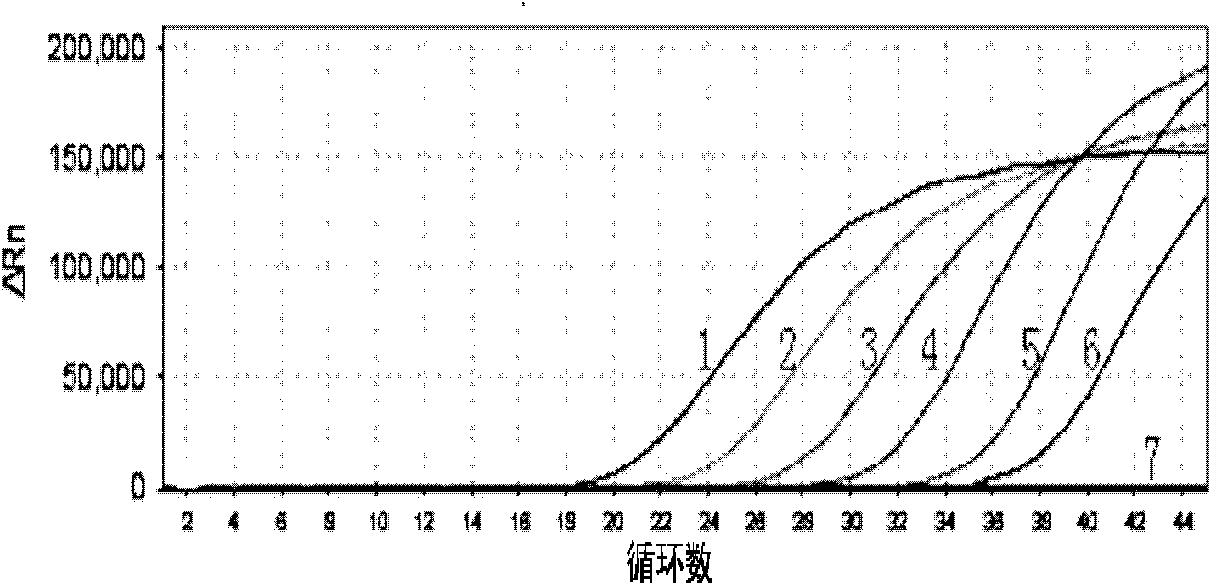
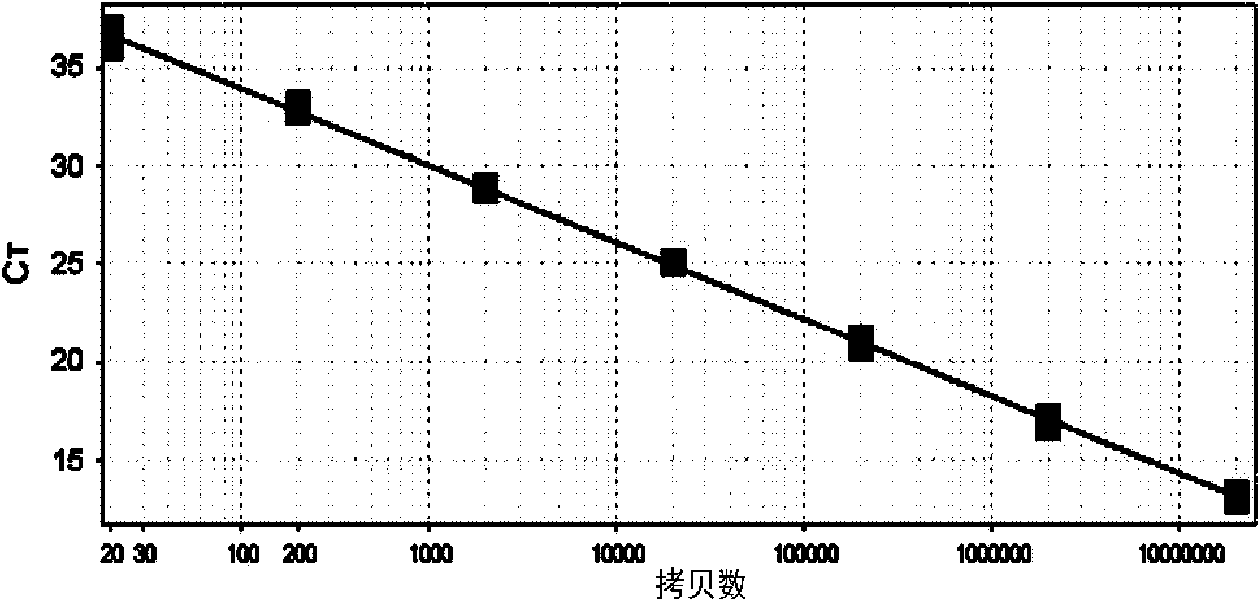
[0030] The components of the Listeria monocytogenes fluorescent quantitative PCR detection kit involved in this embodiment are:
[0031] (both purchased from Treasure Bioengineering (Dalian) Co., Ltd.):
[0032] Fluorescent quantitative PCR reaction solution, fluorescent probe (5μM, 1ul), hot-start TaqDNA polymerase (5U / μl, 0.2ul), standard positive template, negative quality control standard;
[0033] The fluorescent quantitative PCR reaction liquid is specifically: 10×PCR buffer 2ul, 25mM MgCl 21.5ul, 2.5mM dNTPs1.5ul, forward primer (5μM, 1ul), reverse primer (5μM, 1ul); wherein the base sequence of the forward primer is shown in SEQIDNo.1, and the base sequence of the reverse primer is shown in Shown in SEQID No.2;
[0034] The base sequence of the fluorescent probe is shown in SEQID NO: 3, wherein the fluorescent reporter group is FAM, and the fluorescent quencher group is ELIPCE;
[0035] Described standard positive template is the DNA of recombinant plasmid, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com