Rapidly disintegrable Zhenju antihypertensive tablets and preparation method thereof
A technology of Zhenju Jiangya tablet and disintegrant, which is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, and pill delivery, etc. Large, small size of hydrochlorothiazide, etc., to achieve the effects of rapid drug dissolution, improved dissolution rate, and less adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
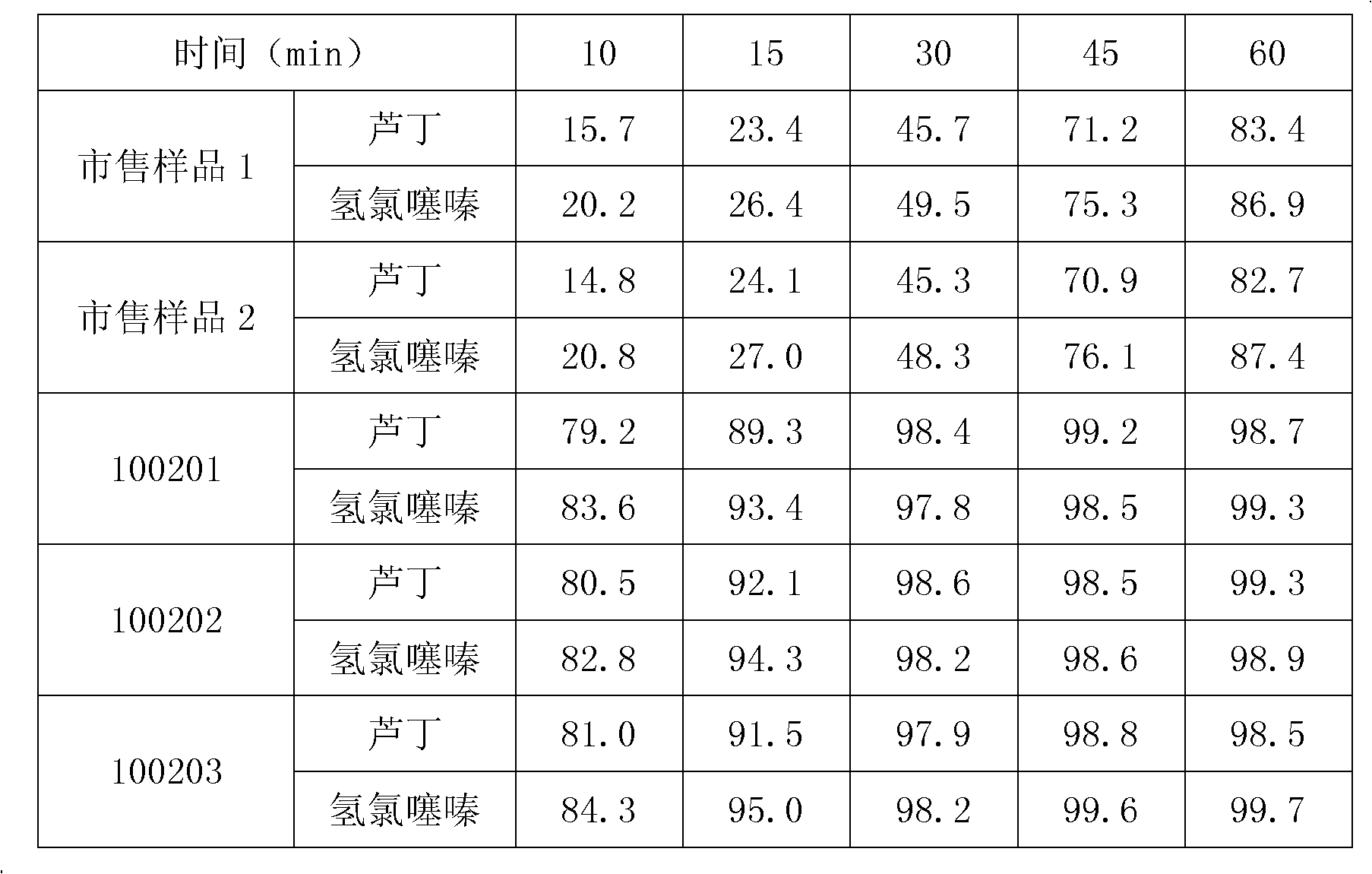
[0037]Embodiment 1: The main ingredient is prepared 1000 Zhenju antihypertensive tablets according to the proportion of wild chrysanthemum flower paste powder 100g, nacre powder 100g, clonidine hydrochloride 30mg, hydrochlorothiazide 5g, rutin 20g and pharmaceutical excipients, and the dosage of each component is as follows :
[0038] Main drug 225.03g
[0039] Sodium carboxymethyl starch (internal addition) 6g
[0040] Cross-linked polyvinylpyrrolidone (internal addition) 12g
[0041] Lactose 40g
[0042] Sodium starch glycolate (additional) 3g
[0043] Cross-linked polyvinylpyrrolidone (additional) 3g
[0044] Sodium Lauryl Sulfate 1g
[0046] 95% ethanol appropriate amount
Embodiment 2
[0047] Embodiment 2: The principal ingredient is prepared 1000 Zhenju antihypertensive tablets according to the proportion of wild chrysanthemum flower cream powder 100g, nacre powder 100g, clonidine hydrochloride 30mg, hydrochlorothiazide 5g, rutin 20g and pharmaceutical excipients, and the dosage of each component is as follows :
[0048] Main drug 225.03g
[0049] Carboxymethyl starch sodium (additional) 8g
[0050] Cross-linked polyvinylpyrrolidone (internal addition) 10g
[0051] 40g pregelatinized starch
[0052] Sodium starch glycolate (extra) 4g
[0053] Cross-linked polyvinylpyrrolidone (additional) 2.5g
[0054] Sodium Lauryl Sulfate 2g
[0056] 95% ethanol appropriate amount
Embodiment 3
[0057] Embodiment 3: The main ingredient is prepared 1000 Zhenju antihypertensive tablets according to the proportion of wild chrysanthemum flower cream powder 100g, nacre powder 100g, clonidine hydrochloride 30mg, hydrochlorothiazide 5g, rutin 20g and pharmaceutical excipients, and the dosage of each component is as follows :
[0058] Main drug 225.03g
[0059] Sodium carboxymethyl starch (internal addition) 7g
[0060] Cross-linked polyvinylpyrrolidone (internal addition) 10g
[0061] Microcrystalline Cellulose 45g
[0062] Sodium starch glycolate (additional) 3.5g
[0063] Cross-linked polyvinylpyrrolidone (additional) 2.5g
[0064] Sodium Lauryl Sulfate 2g
[0066] 95% ethanol appropriate amount
[0067] The preparation method of above-mentioned three embodiments is as follows:
[0068] (a) take by weighing prescription quantity wild chrysanthemum paste powder, nacre powder, rutin and diluent by proportioning, after it is mixed, pulve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com