Method for preparing bentazone
A technology of bentazone and reaction, applied in the field of herbicide preparation, can solve the problems of difficult purification of isopropylaminosulfonyl chloride, unpleasant odor of 2-methylpyridine, increased production cost, etc., and achieves low production cost and high reaction efficiency. The effect of few steps and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
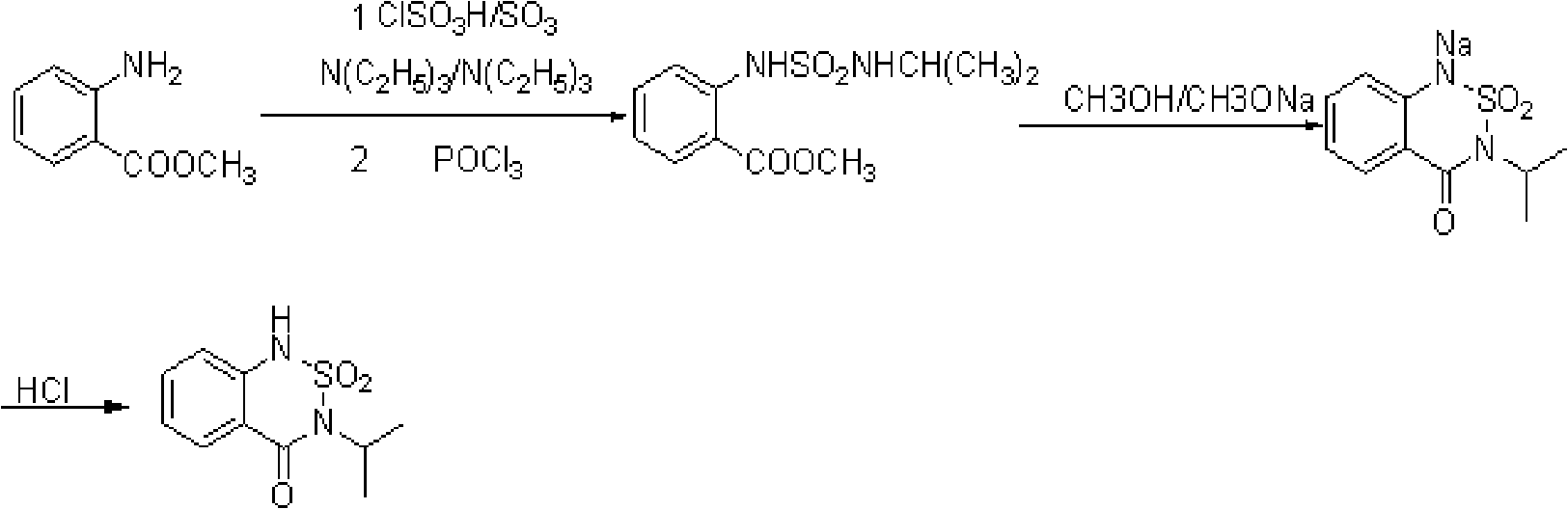
[0019] The preparation method of the bentazone of present embodiment 1 has the following steps:
[0020] ①Add 40g of sulfonating agent sulfur trioxide and 250mL of organic solvent dichloroethane into the reaction flask, cool in an ice bath to -10°C ~ 0°C, slowly add 92.5g of organic base triethylamine dropwise under stirring and Start the reaction of generating sulfur trioxide / triethylamine double salt, stir and keep warm for 0.5h after dropping. Then the temperature was raised to 20°C, and 22.25 g of isopropylamine was added dropwise with stirring, and stirred for 0.3 h after the drop was completed. Then lower the temperature to 10°C, add 54.5g of methyl anthranilate under stirring to start the reaction to generate the corresponding sulfonate, continue to stir for 0.2h after the addition, then raise the temperature to 50°C to 55°C and then stir the reaction 0.3h.
[0021] ②Reduce the temperature of the system obtained in step ① to 15°C to 30°C, add 63g of phosphorus oxychlo...
Embodiment 2
[0025] The rest of this embodiment are basically the same as Example 1, except that the preparation of the intermediate N-methoxycarbonylphenyl-N'-isopropylaminobenzenesulfonamide.
[0026] ①Add 50mL of organic solvent dichloroethane and 18.5g of organic base triethylamine into the reaction bottle, cool in ice bath to -10℃~0℃, add 8g of sulfonating agent sulfur trioxide dropwise under stirring to start For the reaction of generating sulfur trioxide / triethylamine double salt, stir and keep warm for 0.5h after dripping. Then the temperature was raised to 20°C, and 4.43 g of isopropylamine was added dropwise with stirring, and stirred for 0.3 h after the drop was completed. Then lower the temperature to 10°C, add 10.9g of methyl anthranilate under stirring to start the reaction to generate the corresponding sulfonate, continue to stir for 0.2h after the addition, then raise the temperature to 80°C to 85°C and then stir the reaction 0.3h.
[0027] ②Reduce the temperature of the ...
Embodiment 3
[0030] The rest of this embodiment are basically the same as Example 1, except that the preparation of the intermediate N-methoxycarbonylphenyl-N'-isopropylaminobenzenesulfonamide.
[0031]①Add 220g of organic solvent chlorobenzene, 50.5g of organic base triethylamine and 19.3g of isopropylamine into the reaction bottle, then stir at 10℃~0℃ and slowly add 23.3g of sulfonating agent dropwise Chlorosulfonic acid to start the reaction of generating sulfur trioxide / triethylamine double salt, after the drop, the temperature was raised to 10°C, and then 15.1g of methyl anthranilate was added dropwise to start the reaction of generating the corresponding sulfonate , After dropping, stir and react at 50°C to 55°C for 0.5h.
[0032] ②Reduce the temperature of the system obtained in step ① to 15°C to 30°C, add 23.6g of phosphorus oxychloride dropwise, and after the dripping, raise the temperature to 60°C to 65°C and stir to generate N-methoxycarbonylphenyl-N' - Reaction of cumene sulfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com