Ceftizoxime sodium composition sterile powder for injection
A technology of ceftizoxime sodium and sterile powder, which is applied in the field of drug synthesis and preparation, can solve problems such as high cost, inconvenient production operation, and the quality of ceftizoxime sodium does not meet the pharmaceutical standards, so as to reduce production costs and improve the production process. Simple and easy to control, good clinical application effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] [Example 1] Preparation of Crystalline Ceftizoxime Sodium
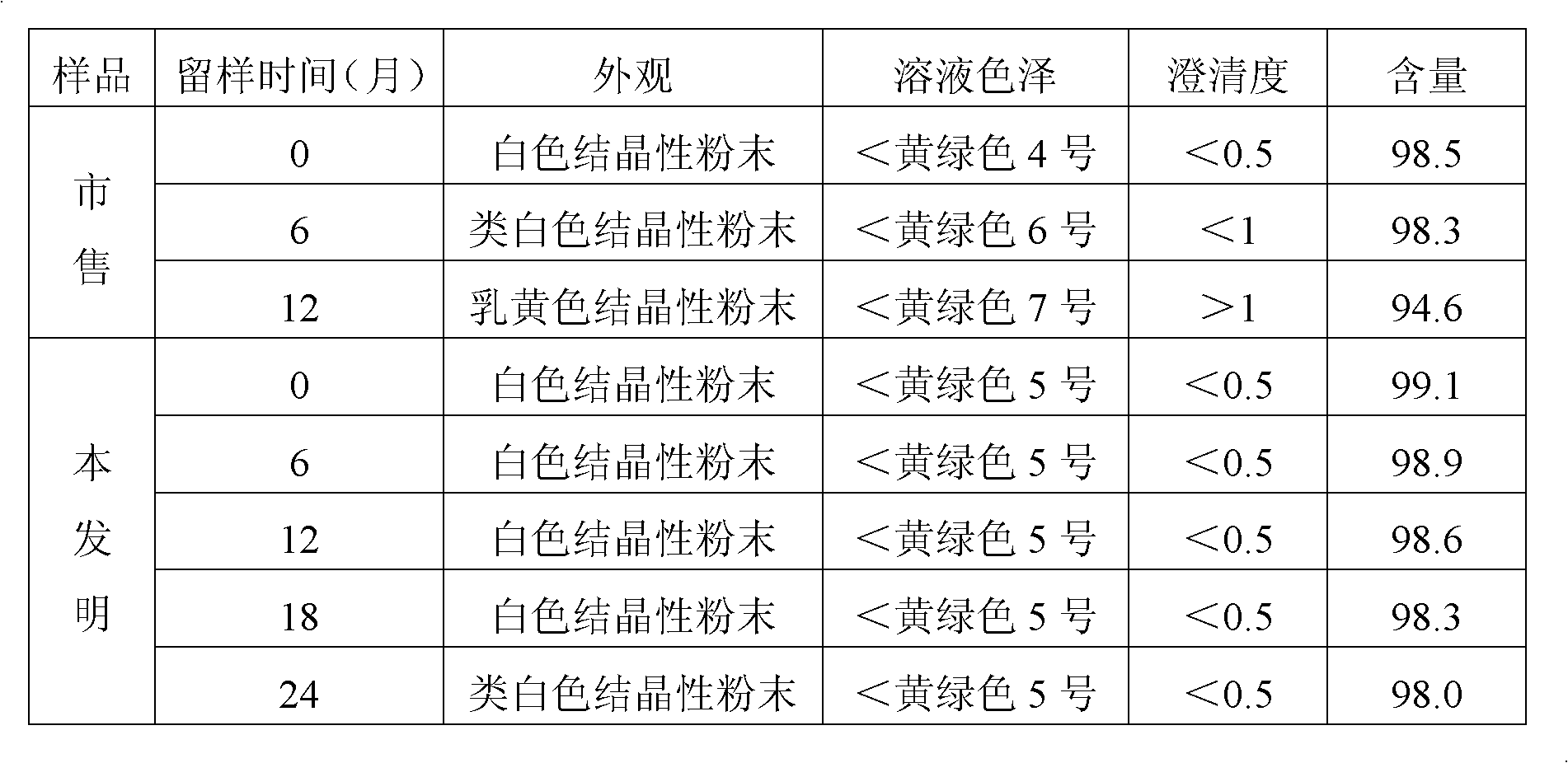
[0041] At room temperature, put 5g of ceftizoxime sodium into the reactor, add 50ml of water to dissolve, stir for 28 minutes, filter, adjust the pH of the filtrate to 6.3 with glacial acetic acid, control the temperature of the filtrate to 18°C, and Under stirring speed, slowly add dropwise the mixed solution of 200ml dehydrated alcohol and acetone (the volume ratio of dehydrated alcohol and acetone is 1: 2) with the speed of 420ml / h to crystallization, then under the stirring of 30 revolutions per minute , control the supersaturated concentration of the solution, continue to add 200ml ethyl acetate slowly, grow crystals, wash twice with a mixed solution of 50ml absolute ethanol and acetone, drain, and dry under reduced pressure to obtain 4.68g of ceftizoxime sodium in crystalline form .
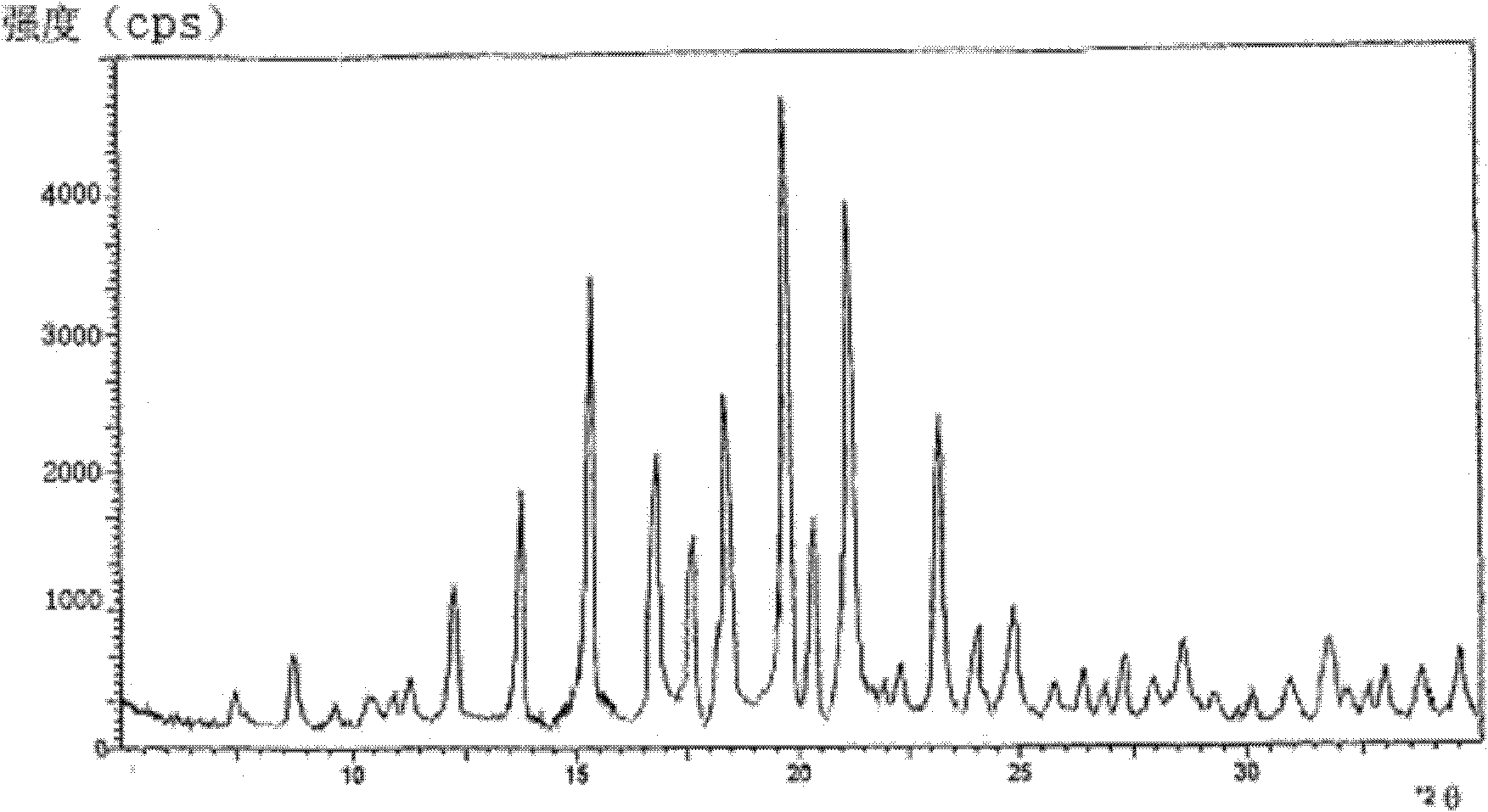
[0042] Use D / max-IIIA DIFFRATOMETER (RIGAKU CORPORATION, JANPAN) X-diffractometer, measure with Cu, K α 1, λ=1.54056A, th...
Embodiment 2
[0043] [Example 2] Preparation of Crystalline Ceftizoxime Sodium
[0044] At room temperature, drop 5g of ceftizoxime sodium into the reactor, add 50ml of water to dissolve, stir for 28 minutes, filter, adjust the pH of the filtrate to 6.9 with glacial acetic acid, control the temperature of the filtrate to 30°C, and Under stirring speed, slowly add dropwise the mixed solution of 200ml dehydrated alcohol and acetone (the volume ratio of dehydrated alcohol and acetone is 1: 4) with the speed of 480ml / h to crystallization, then under the stirring of 70 revolutions per minute , control the supersaturated concentration of the solution, continue to add 600ml ethyl acetate slowly, grow crystals, wash twice with a mixed solution of 50ml absolute ethanol and acetone, then wash twice with 65ml ethyl acetate, drain, and dry under reduced pressure. 4.67 g of ceftizoxime sodium in crystalline form was obtained.
[0045] The X-ray powder diffraction pattern of the obtained crystalline for...
Embodiment 3
[0046] [Example 3] Preparation of Crystalline Ceftizoxime Sodium
[0047] At room temperature, put 5g of ceftizoxime sodium into the reactor, add 50ml of water to dissolve, stir for 28 minutes, filter, adjust the pH of the filtrate to 6.5 with glacial acetic acid, control the temperature of the filtrate to 25°C, and Under stirring speed, slowly add dropwise the mixed solution of 200ml dehydrated alcohol and acetone (the volume ratio of dehydrated alcohol and acetone is 1: 3) with the speed of 450ml / h to crystallization, then under the stirring of 50 revolutions per minute , control the supersaturated concentration of the solution, continue to add 400ml ethyl acetate slowly, grow crystals, wash twice with 65ml ethyl acetate, drain, and dry under reduced pressure to obtain 4.66g of ceftizoxime sodium in crystalline form.
[0048] The X-ray powder diffraction pattern of the obtained crystalline form of ceftizoxime sodium is consistent with that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com