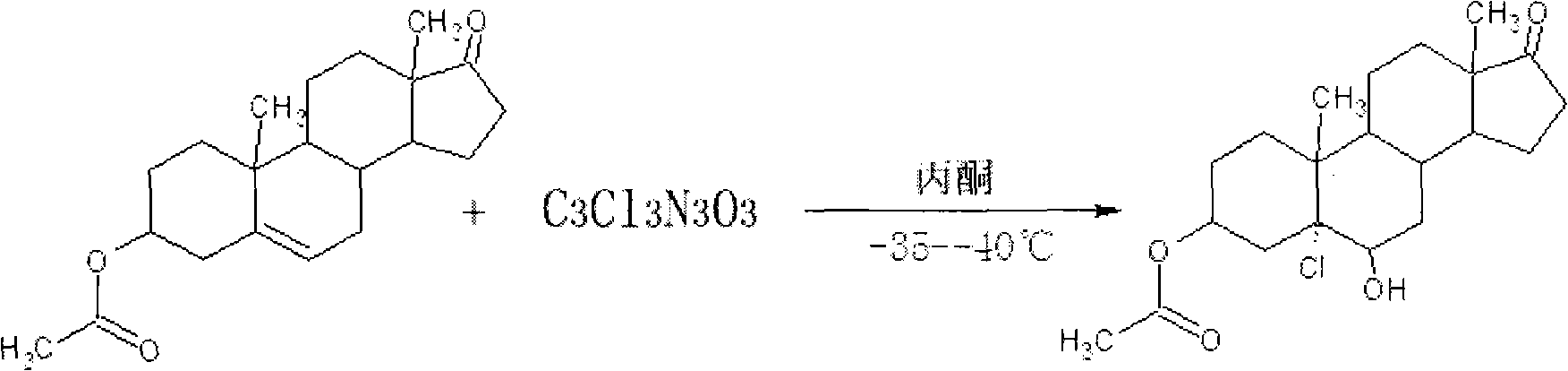Preparation method of 19-nor-4-androstenedione
A technology of androstenedione and demethylation, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of low product reaction yield, unsuitability for large-scale production, low yield of alkali dereaction, etc., and achieve high product purity, The effect of low cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of 19-desmethyl-4-androstenedione, the preparation process mainly consists of three parts: addition reaction, decarboxylation oxide purification, and alkali dereaction. The specific preparation method and steps are as follows:
[0033] 1) Addition reaction: In a 100ml three-necked flask, add 56g of acetone and 4g of rearrangements, stir to dissolve, turn on the refrigerator, cool to -34°C, add 6g of drinking water, and keep between -35--40°C, Add (1.2g trichloroisocyanuric acid + acetone 8g) solution dropwise, stir and keep warm for 45 minutes, pour into the pre-prepared (12g water + 2g sodium bisulfite) solution, stir, concentrate, add 80g drinking water after concentration, and pump Filter to obtain 3.3 g (82%) of off-white powder of the adduct, area content: 98.14%, purity (external standard method) 90.33%, MP: 217°C.
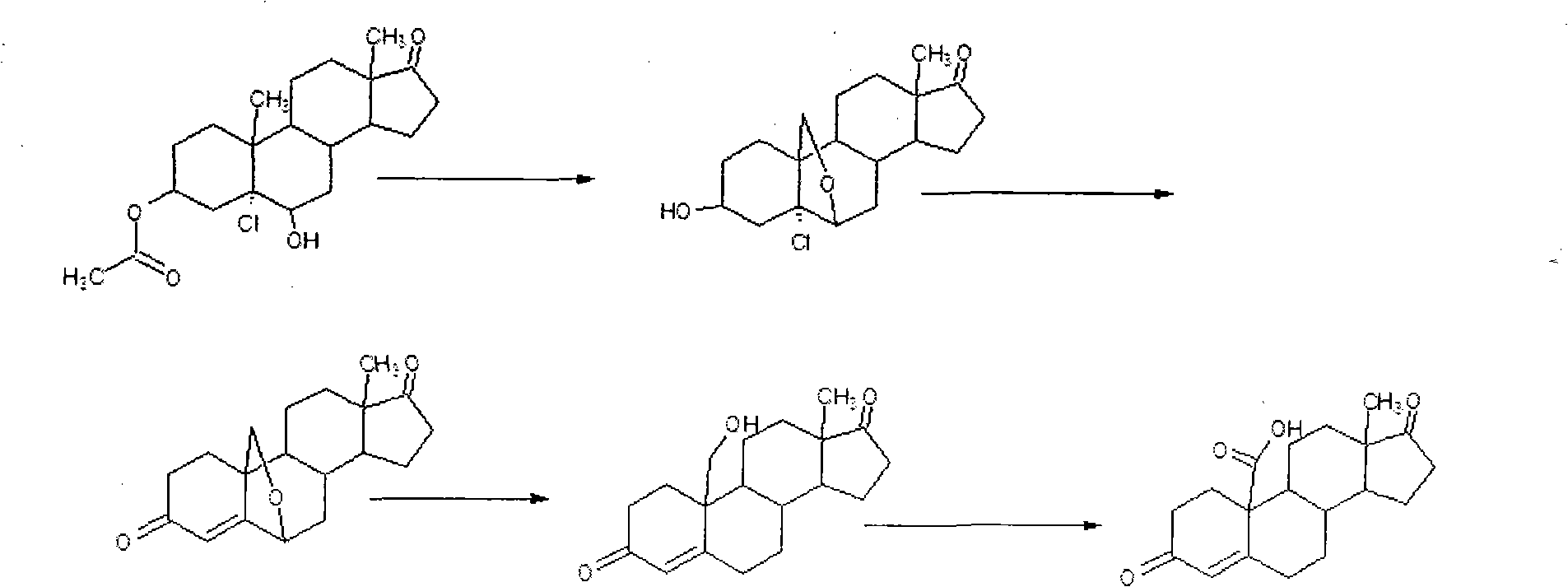
[0034] 2) Purification of decarboxylation oxides: In a 250ml three-necked flask, add 195g of water and 9.9g of sodium bicarbonat...
Embodiment 2
[0037] A preparation method of 19-desmethyl-4-androstenedione, the preparation process mainly consists of three parts: addition reaction, decarboxylation oxide purification, and alkali dereaction. The specific preparation method and steps are as follows:
[0038] 1) Addition reaction: In a 500ml three-necked flask, add 280g of acetone and 20g of rearrangement, stir to dissolve, turn on the refrigerator, cool to -34°C, add 30g of drinking water, between -35--40°C, Add (6g of trichloroisocyanuric acid + 40g of acetone) solution dropwise, stir and keep warm for 45 minutes, pour into the pre-prepared (60g of water + 10g of sodium bisulfite) solution, stir, concentrate, add 400g of drinking water after concentration, and filter with suction 17g (85%) of off-white powder of the adduct was obtained, the area content: 99.10%, the purity (external standard method) was 91.43%, MP: 221°C.
[0039] 2) Purification of decarboxylation oxides: In a 500ml three-necked flask, add 390g of water...
Embodiment 3
[0042] A preparation method of 19-desmethyl-4-androstenedione, the preparation process mainly consists of three parts: addition reaction, decarboxylation oxide purification, and alkali dereaction. The specific preparation method and steps are as follows:
[0043] 1) Addition reaction:
[0044] In a 1000ml three-neck flask, add 560g of acetone, 40g of rearrangement, stir to dissolve, turn on the freezer, cool to -34°C, add 60g of drinking water, drop (trichloroiso Cyanuric acid 12g + acetone 80g) solution, stirred and kept warm for 45 minutes, poured into the pre-prepared (120g water + 20g sodium bisulfite) solution, stirred, concentrated, concentrated and dried, added 800g drinking water, and suction filtered to obtain the adduct Off-white powder 34g (85%), area content: 98.87%, purity (external standard method) 92.27%, MP: 219°C.
[0045] 2) Purification of decarboxylation oxides: In a 1000ml three-necked flask, add 780g of water and 39.6g of sodium bicarbonate, stir to comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com