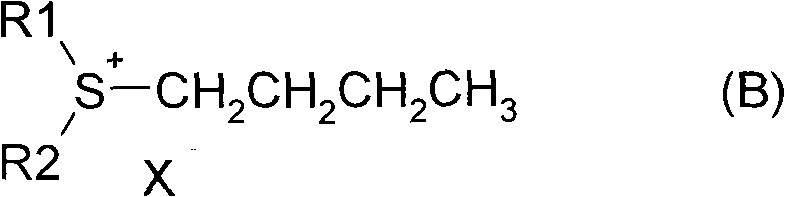Method for synthesizing 1,2-pentanediol from n-butyl alcohol
A technology of pentanediol and n-butanol, which is applied in the field of synthesis of fine chemical intermediates, to achieve the effects of safe production process, simplified separation procedure and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
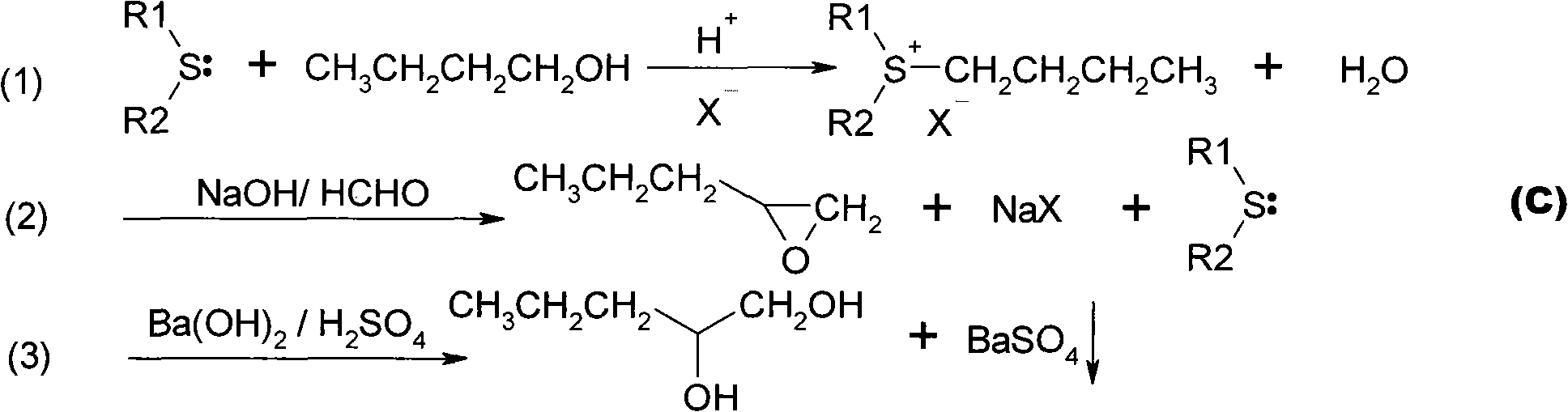
[0019] In a 1000ml three-necked flask, add 120g of diisopropyl sulfide (1mol) and 150g of n-butanol, after heating to 80-100 degrees, slowly add 120g of 85% sulfuric acid dropwise, and keep it at 100-120 degrees for 3~ After 4 hours, the layers were separated after resting to obtain 265 g of the sulfenyl salt solution in the lower layer. Mix sulfenyl salt solution with 160 g of 37% formaldehyde solution, add dropwise 40% liquid caustic soda NaOH until the pH is greater than 13, react at 30-40 degrees for 3-4 hours, and obtain a light yellow oil layer, which is 1,2-epoxypentyl A mixture of alkanes and diisopropyl sulfide.
[0020] Add 5g of barium hydroxide to 600g of 85%wt methanol aqueous solution, stir evenly, and heat to reflux, then slowly add the above oil layer mixture dropwise, after the dropwise addition, continue to reflux for 2h, then dropwise add 20%wt of sulfuric acid to neutralize to pH = 7.0, the barium sulfate precipitate was removed by filtration to obtain a s...
Embodiment 2
[0022] In a 1000ml three-neck flask with a condensing reflux tube, add 62g dimethyl sulfide (1mol) and 150g n-butanol, after heating to reflux, slowly add 120g of 85% sulfuric acid dropwise, keep the reflux reaction for 3-4h, after standing still The layers were separated to obtain 168 g of the lower layer sulfur salt solution. Mix sulfenyl salt solution with 100 g of 37% formaldehyde solution, add dropwise 40% liquid caustic soda NaOH until the pH is greater than 13, react at 30-40 degrees for 3-4 hours, and obtain a light yellow oil layer, which is 1,2-epoxypentyl A mixture of alkanes and dimethyl sulfide.
[0023] Add 5g of barium hydroxide to 600g of 85%wt methanol aqueous solution, stir evenly, and heat to reflux, then slowly add the above oil layer mixture dropwise, after the dropwise addition, continue to reflux for 2h, then dropwise add 20%wt of sulfuric acid to neutralize to pH = 7.0, the barium sulfate precipitate was removed by filtration to obtain a salt-free 1,2-...
Embodiment 3
[0025] In a 1000ml three-necked flask with a condensing reflux tube, add 150g of dibutyl sulfide (1mol) and 150g of n-butanol. After heating and keeping at 80-100 degrees, slowly add 120g of 85% sulfuric acid dropwise, and keep the reflux reaction for 3 After ~4h, the layers were separated after resting to obtain 352g of the lower layer of sulfuric acid salt solution. Mix sulfenyl salt solution with 100 g of 37% formaldehyde solution, add dropwise 40% liquid caustic soda NaOH until the pH is greater than 13, react at 30-40 degrees for 3-4 hours, and obtain a light yellow oil layer, which is 1,2-epoxypentyl A mixture of alkanes and dibutyl sulfide.
[0026] Add 5g of barium hydroxide to 600g of 85%wt methanol aqueous solution, stir evenly, and heat to reflux, then slowly add the above oil layer mixture dropwise, after the dropwise addition, continue to reflux for 2h, then dropwise add 20%wt of sulfuric acid to neutralize to pH = 7.0, the barium sulfate precipitate was removed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com