Preparation method of sulfonanilide compound
A sulfonanilide and sulfonanilide technology are applied in the field of preparation of sulfonanilide compounds, and can solve the problems of expensive catalyst, uneconomical, low yield and the like, and achieve good reaction effect, few side reactions and low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
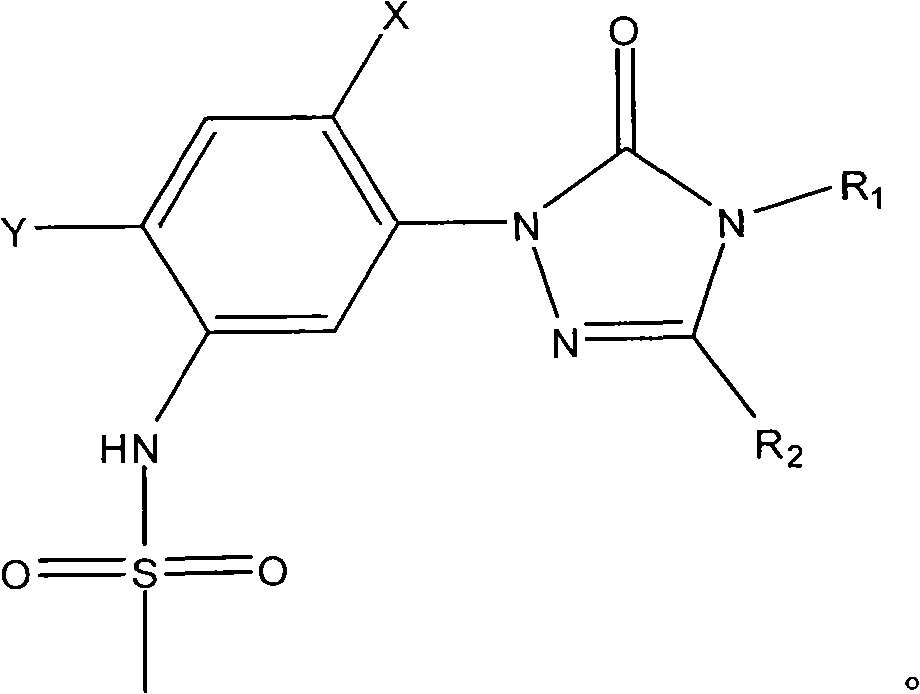
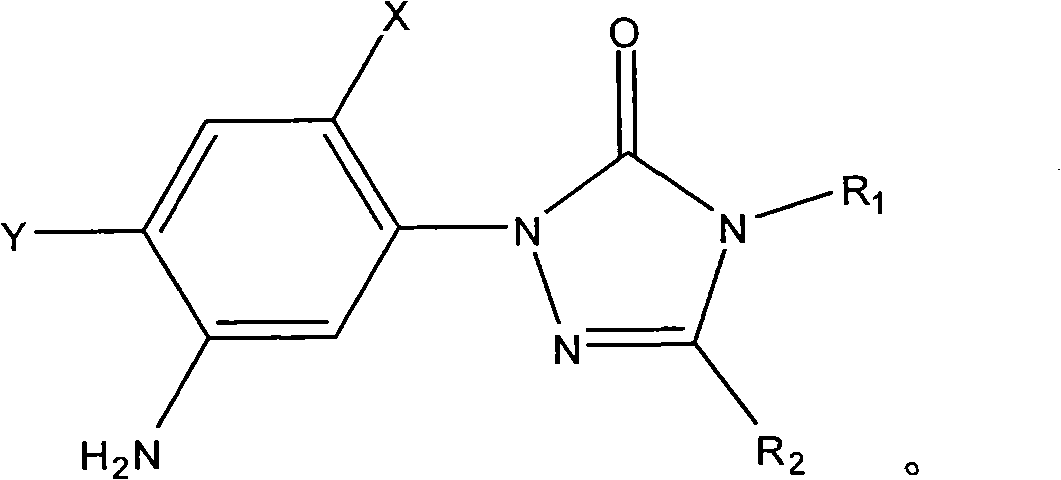
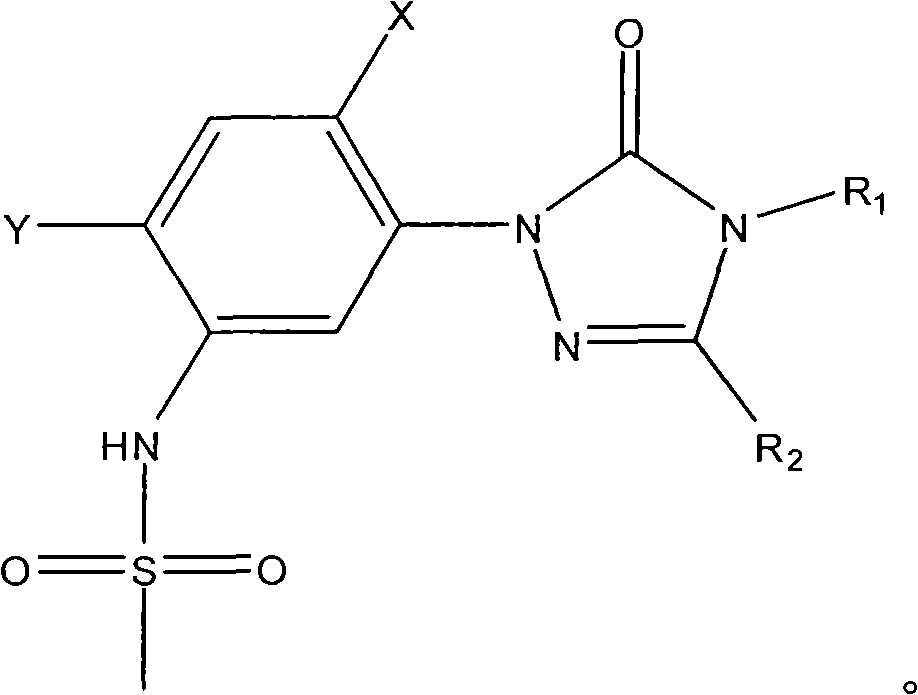
[0018] Add 0.1 mole of 1-(5-amino-2,4-dichlorophenyl)-4-difluoromethyl-3-methyl-1 hydrogen-1,2,4-triazole- 5(4 hydrogen) ketone, 0.1 mole of toluene, heated to 100°C with stirring, added 0.01 mole of triphenylphosphine, then added dropwise 0.12 mole of methanesulfonyl chloride, after the dropwise addition, kept the system temperature at 100°C, and reacted After 12 hours, stop the reaction, cool, filter with suction, wash and dry to obtain N-(2,4-dichloro-5-(4-difluoromethyl-4,5-dihydro-3-methyl-5 -Oxo-1H-1,2,4-triazol-1-yl)phenyl)methanesulfonamide, yield 87%, content 93%. N-(2,4-dichloro-5-(4-difluoromethyl-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazole-1- base) phenyl) methanesulfonamide 1 HNMR (500MHz, (CD 3 ) 2 SO, TMS) 69.81 (1H, s), 7.98 (1H, s), 7.70 (1H, s), 7.52 (1H, t, J=57Hz), 3.13 (3H, s), 2.41 (3H, s); MS ([M-H] - ): 384.8.
Embodiment 2
[0020] Add 0.1 mole of 1-(5-amino-2,4-dichlorophenyl)-3,4-dimethyl-1hydrogen-1,2,4-triazole-5(4hydrogen ) ketone, 1 mole of toluene, heated to 90°C under stirring, added 0.001 mole of tributylphosphine, then added dropwise 1 mole of methanesulfonyl chloride, after the dropwise addition, kept the system temperature at 90°C, and stopped after 25 hours of reaction Reaction, cooling, suction filtration, washing, and drying to obtain N-(2,4-dichloro-5-(3,4-dimethyl-4,5-dihydro-5-oxo-1H-1, 2,4-triazol-1-yl)phenyl)methanesulfonamide, yield 79%, content 94%.
Embodiment 3
[0022] Add 0.1 mole of 1-(5-amino-2,4-dichlorophenyl)-4-difluoromethyl-1 hydrogen-1,2,4-triazole-5(4 hydrogen) into the three-necked flask Ketone, 0.3 mole of xylene, heat up to 136°C with stirring, add 0.1 mmol of triethyl phosphite, then dropwise add 0.1 mole of methanesulfonyl chloride, after the dropwise addition, keep the system temperature at 136°C, and react for 20 hours Then stop the reaction, cool, filter with suction, wash, and dry to obtain N-(2,4-dichloro-5-(4-difluoromethyl-4,5-dihydro-5-oxo-1 hydrogen- 1,2,4-Triazol-1-yl)phenyl)methanesulfonamide, yield 83%, content 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com