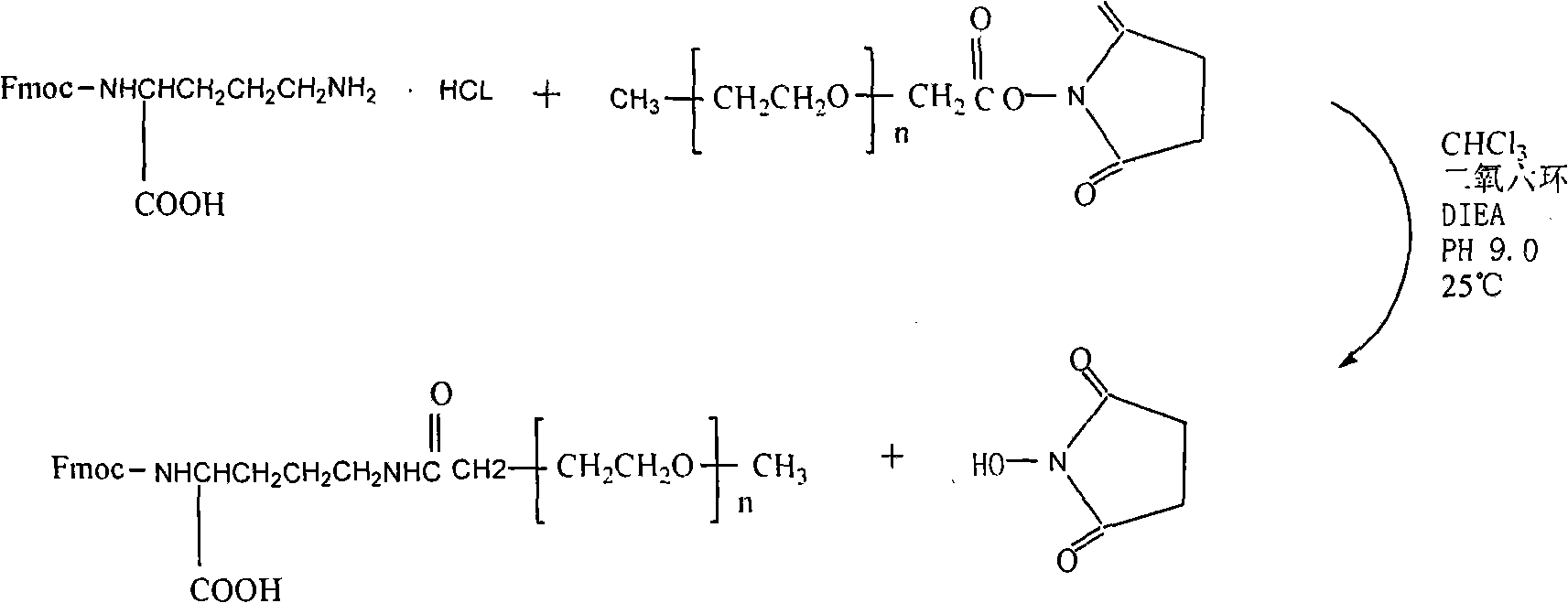Method for synthesizing mono pegylation-thymopentin by solid phase and liquid phase combination
A technology of PEGylation and solid-phase synthesis, which is applied in chemical instruments and methods, carrier binding/immobilization of peptides, peptides, etc., to achieve the effect of accurate structure, good mass production basis, and mature synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
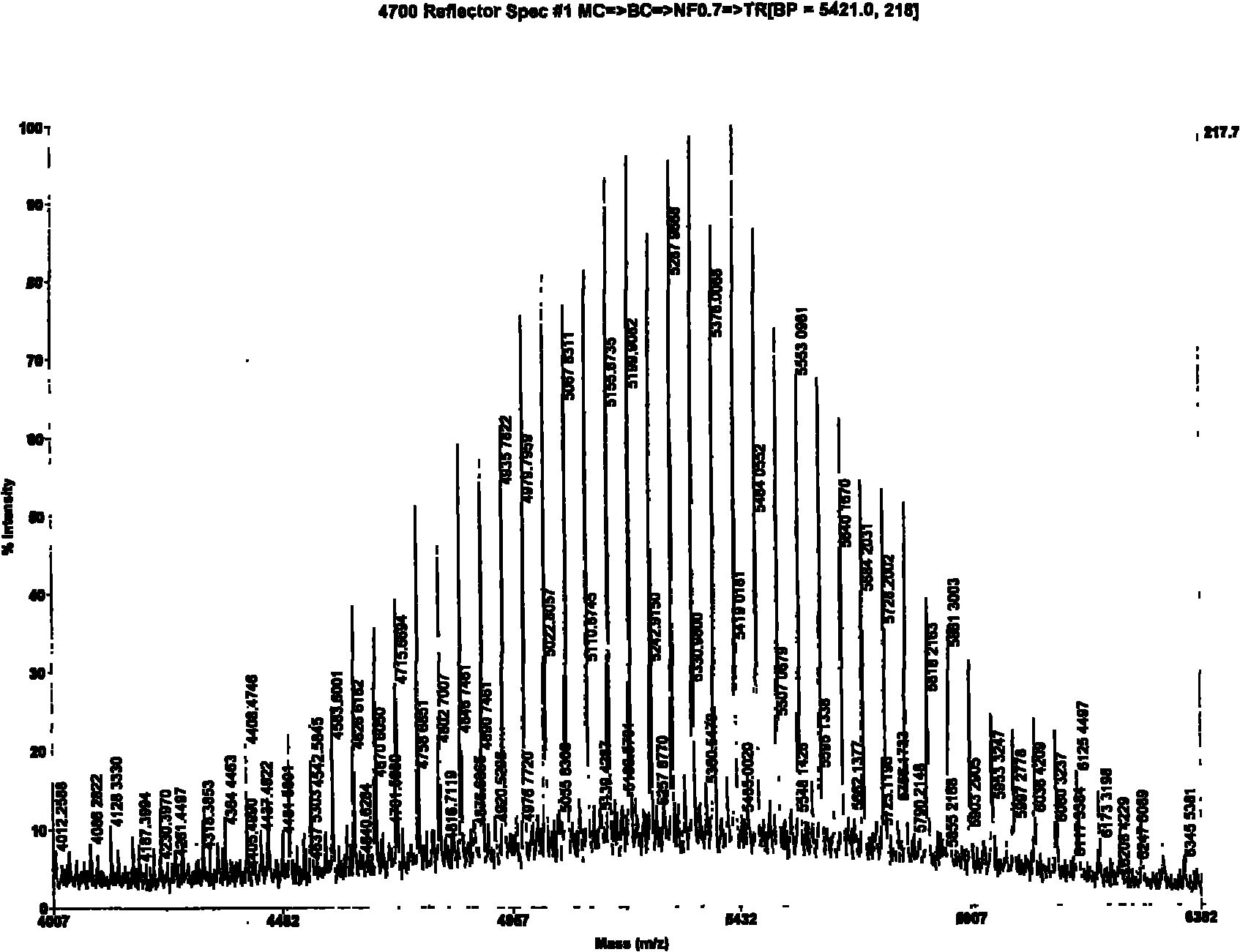
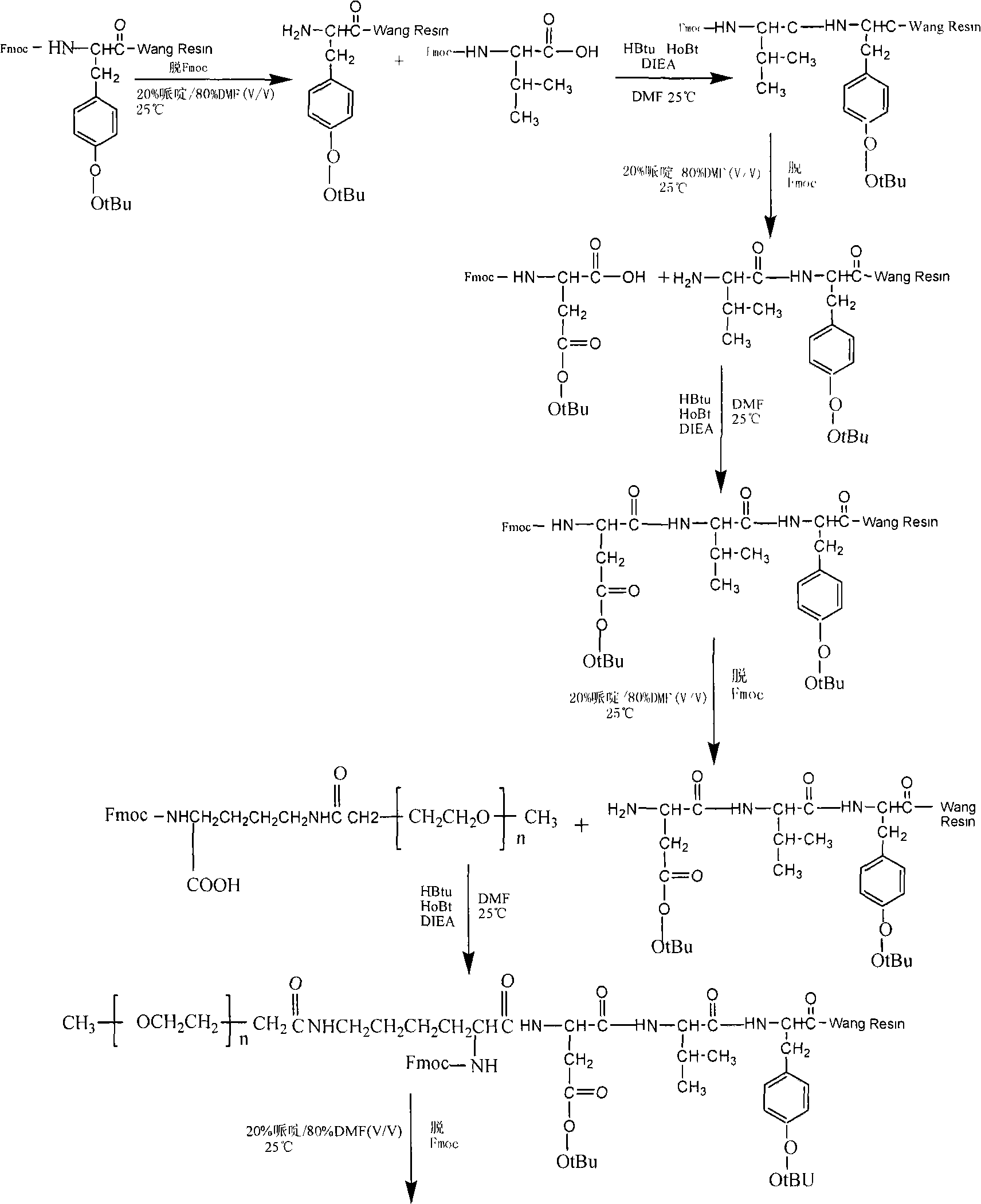
[0022] A method for synthesizing PEGylated Thymopentin with a solid-liquid phase method, the steps of which are divided into:
[0023] a. The monomethoxypolyethylene glycol modification (mPEG-SCM) was connected to the side chain amino group of α-amino-protected lysine (ε-NH 2 ); 400mg of fluorenylmethoxycarbonyl-protected lysine hydrochloride (Fmoc-lys-OH.HCl) reacted with 700mg of monomethoxypolyethylene glycol modification (mPEG-SCM), and the reaction system was 6ml of chloroform and 24ml of dioxane; 800 μl of diisopropylethylamine (N, N-Diisopropylsilane, referred to as DIEA) was added dropwise at room temperature to keep the pH at 9.0, stirred by magnetic force, and reacted overnight; Add 40mL of water to dissolve, filter out water-insoluble matter, dialyze with a dialysis bag (MW, 3500) for 48 hours, and freeze-dry to obtain 600mg of white powder (Fmoc-lys(PEG)-OH), with a yield of 78.5%.
[0024] The NMR data of the product are as follows:
[0025] 1 H-NMR (DMSO) 7.31...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com