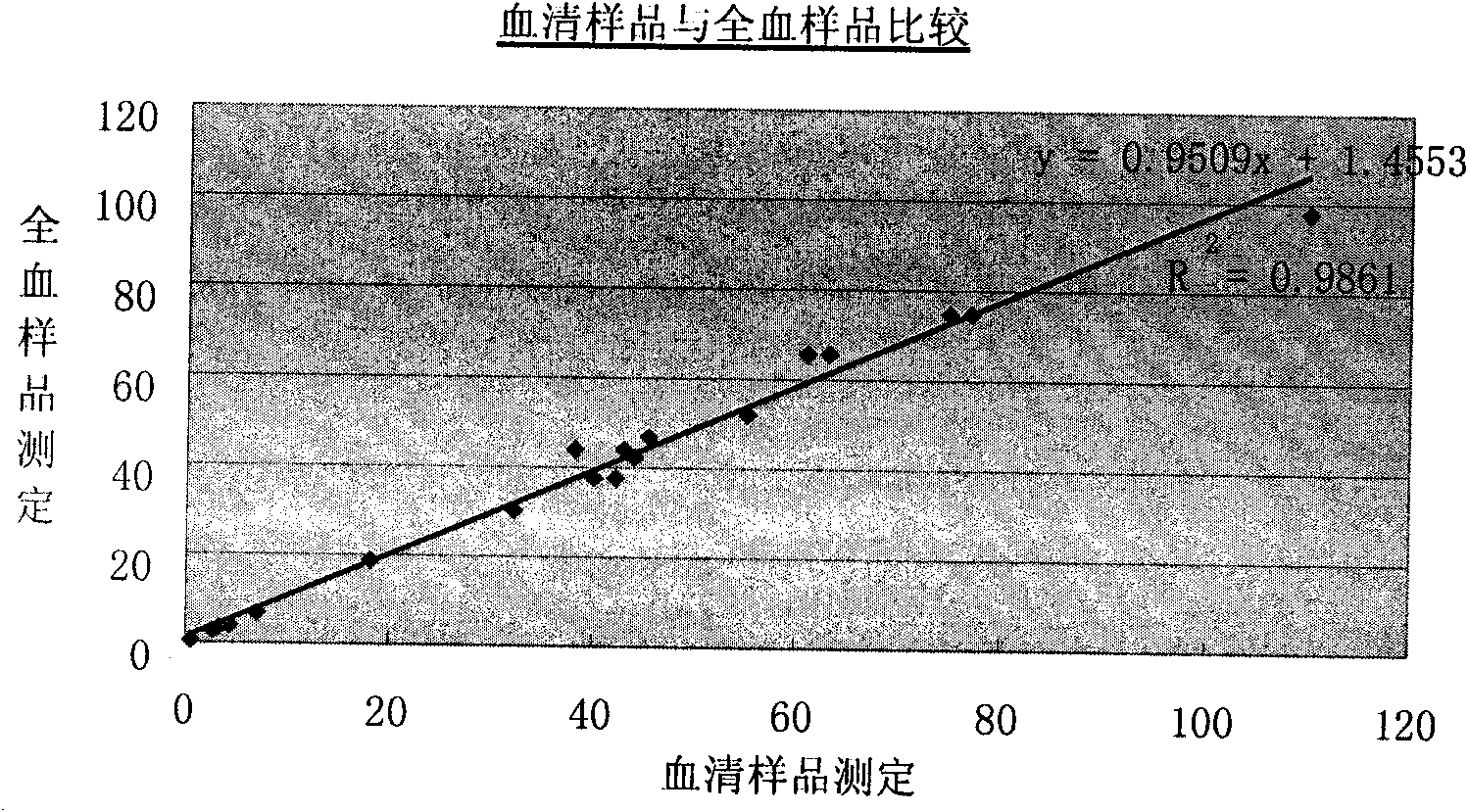
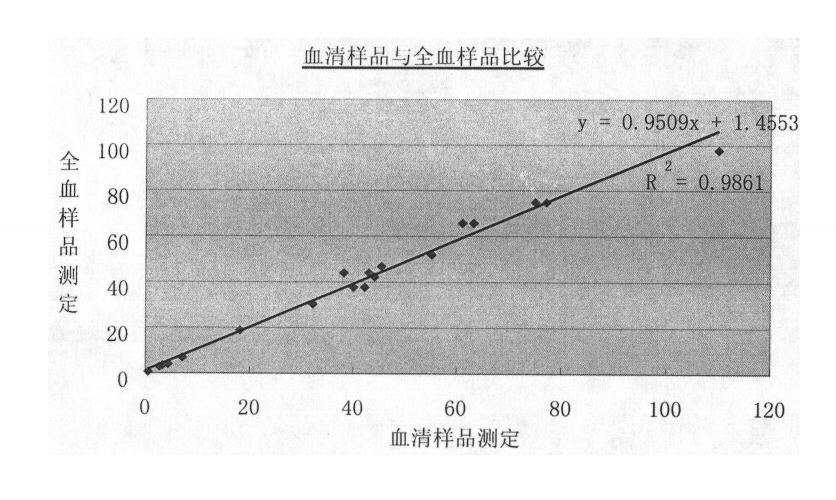
Reagent kit for measuring content of C-reactive protein in whole blood and measuring method thereof
The technology of a reactive protein and a determination method is applied to a kit for determining the content of C-reactive protein in whole blood and the field of determination thereof, and achieves the effects of simple operation, reduced pain and accurate results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The C-reactive protein content determination kit in whole blood of the present invention comprises reagent 1 and reagent 2, and reagent 1 comprises surfactant triton 0.1g / L, stabilizer bovine serum albumin 0.5g / L, preservative 0.05 g / L and HEPES buffer (100mmol / L, PH7.4); Reagent 2 includes 0.5g / L polystyrene latex adsorbed with CRP antibody, stabilizer egg albumin 0.5g / L, preservative 0.05g / L and PH7.4 HEPES buffer 100mmol / L.
[0033] The method for measuring C-reactive protein content in whole blood of the present invention comprises the following steps
[0034] A. Collect the peripheral blood of the subject, which is a whole blood sample;
[0035] B. Incubate 5ul of the whole blood sample with 200ul of the above reagent 1 at 37°C and mix for 30 seconds to dissolve the red blood cells in the sample;
[0036] C. Add 200ul of reagent 2 equilibrated to room temperature to the solution in step B and mix well, so that the C-reactive protein in the sample and the C-react...
Embodiment 2
[0039] The C-reactive protein content determination kit in whole blood of the present invention comprises reagent 1 and reagent 2, and reagent 1 comprises surfactant saponin 3g / L, stabilizer polyamino acid 5g / L, preservative 0.5g / L and HEPES buffer (100mmol / L, PH7.4); Reagent 2 includes polystyrene latex that 3g / L is adsorbed with CRP antibody, stabilizer skimmed milk powder 5g / L, preservative 0.5g / L and HEPES buffer ( 100mmol / L, pH7.4).
[0040] The method for measuring C-reactive protein content in whole blood of the present invention comprises the following steps
[0041] A. Collect the peripheral blood of the subject as a whole blood sample;
[0042] B. Mix 5 ul of the whole blood sample with 200 ul of the above reagent 1 incubated at 37°C for 30 seconds to dissolve the red blood cells in the sample;
[0043] C. Add 200ul of reagent 2 equilibrated to room temperature to the solution in step B and mix well, so that the C-reactive protein in the sample and the C-reactive p...
Embodiment 3
[0046]The C-reactive protein content determination kit in whole blood of the present invention comprises reagent 1 and reagent 2, and reagent 1 comprises surfactant cetylamino ammonium bromide 1g / L, stabilizer bovine serum albumin 1g / L, Stabilizer egg albumin 1g / L, preservative 0.1g / L and HEPES buffer (100mmol / L, pH7.4); Reagent 2 includes 2g / L polystyrene latex adsorbed with CRP antibody, stabilizer cattle Serum albumin 1g / L, preservative 0.1g / L and HEPES buffer (100mmol / L, PH7.4).
[0047] The method for measuring C-reactive protein content in whole blood of the present invention comprises the following steps
[0048] A. Collect the peripheral blood of the subject as a whole blood sample;
[0049] B. Incubate 5ul of the whole blood sample with 200ul of the above reagent 1 at 37°C and mix for 30 seconds to dissolve the red blood cells in the sample;
[0050] C. Add 200ul of reagent 2 equilibrated to room temperature to the solution in step B and mix well, so that the C-reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com