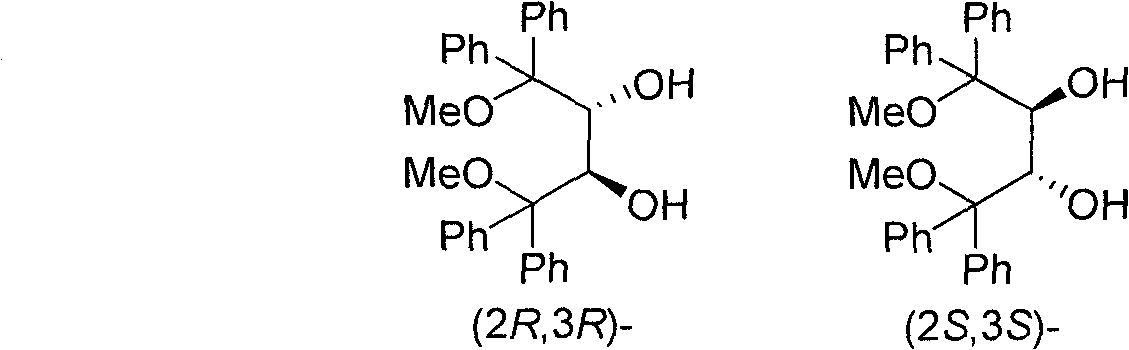
Preparation method of (2R,3R)-1,4-dimethoxyl-1,1,4,4-tetraphenyl-2,3-butanediol and (2S,3S)-1,4-dimethoxyl-1,1,4,4-tetraphenyl-2,3-butanediol
A dimethoxy, tetraphenyl technology, applied in the field of chiral compound preparation chemistry, to achieve the effects of high product yield, easy operation, easy protection and deprotection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of enantiopure 1,1,4,4-tetraphenyl substituted butanetetraol
[0029] According to the conventional reaction method, the enantiomerically pure (2R, 3R)- or (2S, 3S)-diethyl tartrate solution in tetrahydrofuran was added dropwise to four to ten equivalents of phenyl magnesium bromide in tetrahydrofuran solution. . After the reaction is completed, it is quenched with saturated aqueous ammonium chloride solution, extracted with ether, and (2R, 3R)- or (2S, 3S)-1,1,4,4-tetraphenylbutanetetraol is separated from the organic phase . After silica gel column chromatography, the yield of (2R, 3R)- or (2S, 3S)-1,1,4,4-tetraphenyltetramole is about 50%.
[0030] (2S,3S)-1,1,4,4-tetraphenyltetramethylenetetraol: m.p.: 149-150°C; [α] D 25 =-156.6(c=1.1, in CHCl 3 ).IR: 3437, 3058, 2916, 1598, 1492, 1447, 1062, 698. 1 H-NMR: 7.37-7.13 (m, 20H, Ar-H), 4.63 (d, 2H, J=4.8 Hz, OH, add D 2 Disappear after O), 4.42 (d, 2H, J = 4.5 Hz, CH); 3.69 (d, 2H, J = 4.8, OH, add ...
Embodiment 2
[0032] Example 2: Preparation of Chiral Spiroborate Sodium Salt
[0033] Add 1,1,4,4-tetraphenyltetramethylenetetraol and THF into a two-neck round bottom flask, stir to dissolve, and then add H 3 BO 3 , NaOH and water, stir to dissolve, heat to reflux until all the raw material 1,1,4,4-tetraphenylbutanetetraol disappears, remove the solvent THF by rotary evaporation, treat with water, filter with suction, and crystallize the solid with ethanol to obtain chiral spiroboronic acid Sodium, the yield is 96%, mp>300℃, [α] D 25 = +10.79 (c 1.0, THF). 1 H NMR(300MHz, DMSO-d 6 ): δ7.15-6.85(m, 32H, 32Ph-H), 6.76(s, 8H), 4.46(s, 4H, 4OC-H), 4.18(s, 4H, 4O-H, add D 2 Disappear after O). 13 C NMR(75MHz, DMSO-d 6 ): δ153.2, 148.9, 133.2, 132.7, 131.8, 131.0, 85.6, 83.0. Ms: 883(M+1) + .
Embodiment 3
[0034] Example 3: Preparation of Chiral Spiroborate Sodium Salt
[0035] 1,1,4,4-Tetraphenylbutanetetraol and sodium borohydride were refluxed in THF until all the tetraols of the raw materials disappeared, and then spin-dried to obtain a white solid, which was stirred with water and filtered. The solid was crystallized with ethanol. The yield was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com