Preparation method of cefathiamidine
A technology for cefathiamidine and aminocephalosporanic acid is applied in the field of preparation of chemical raw material cefathiamidine, and can solve the problems of prolonged operation time, overflow phenomenon, unsatisfactory yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
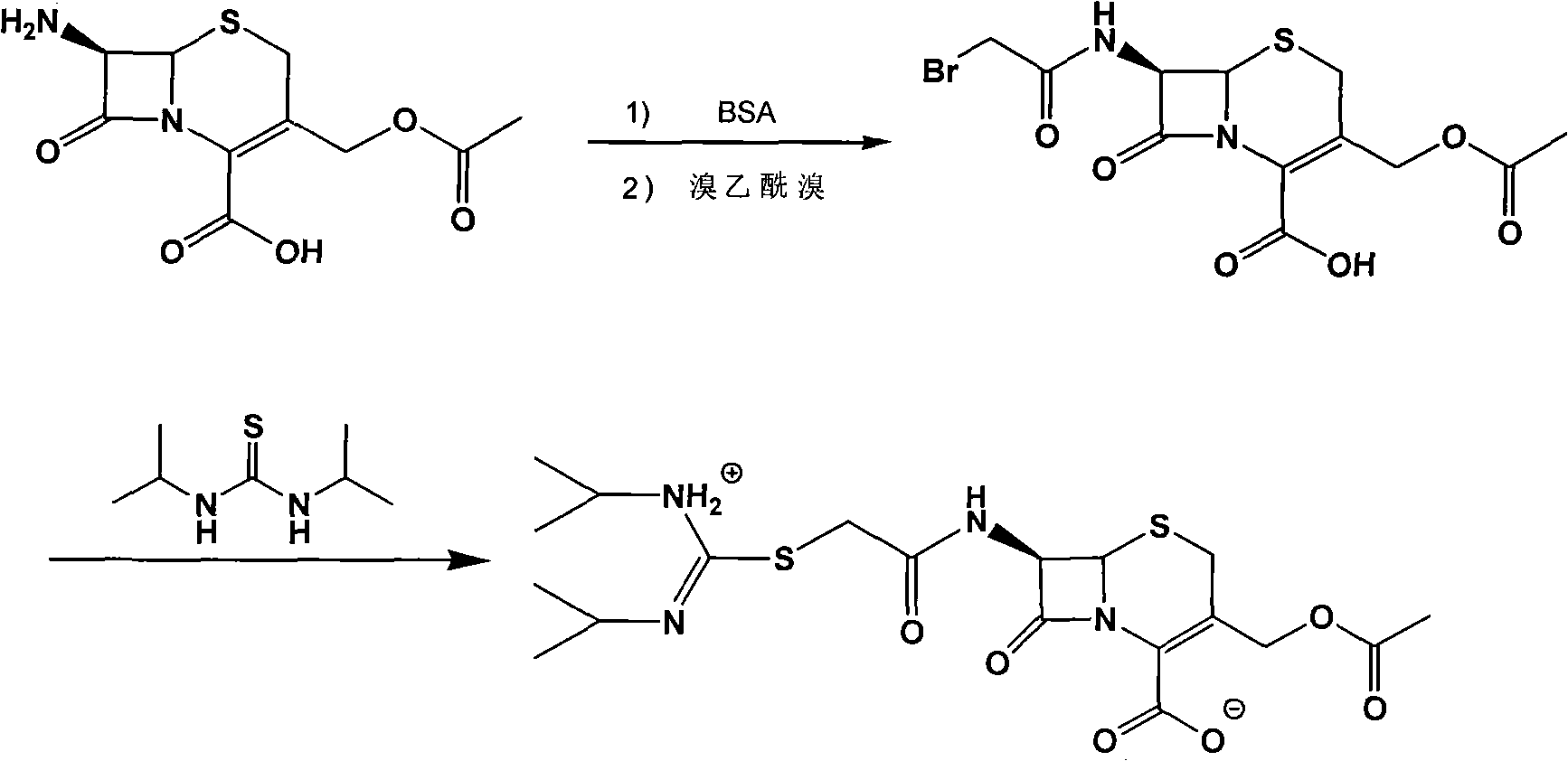
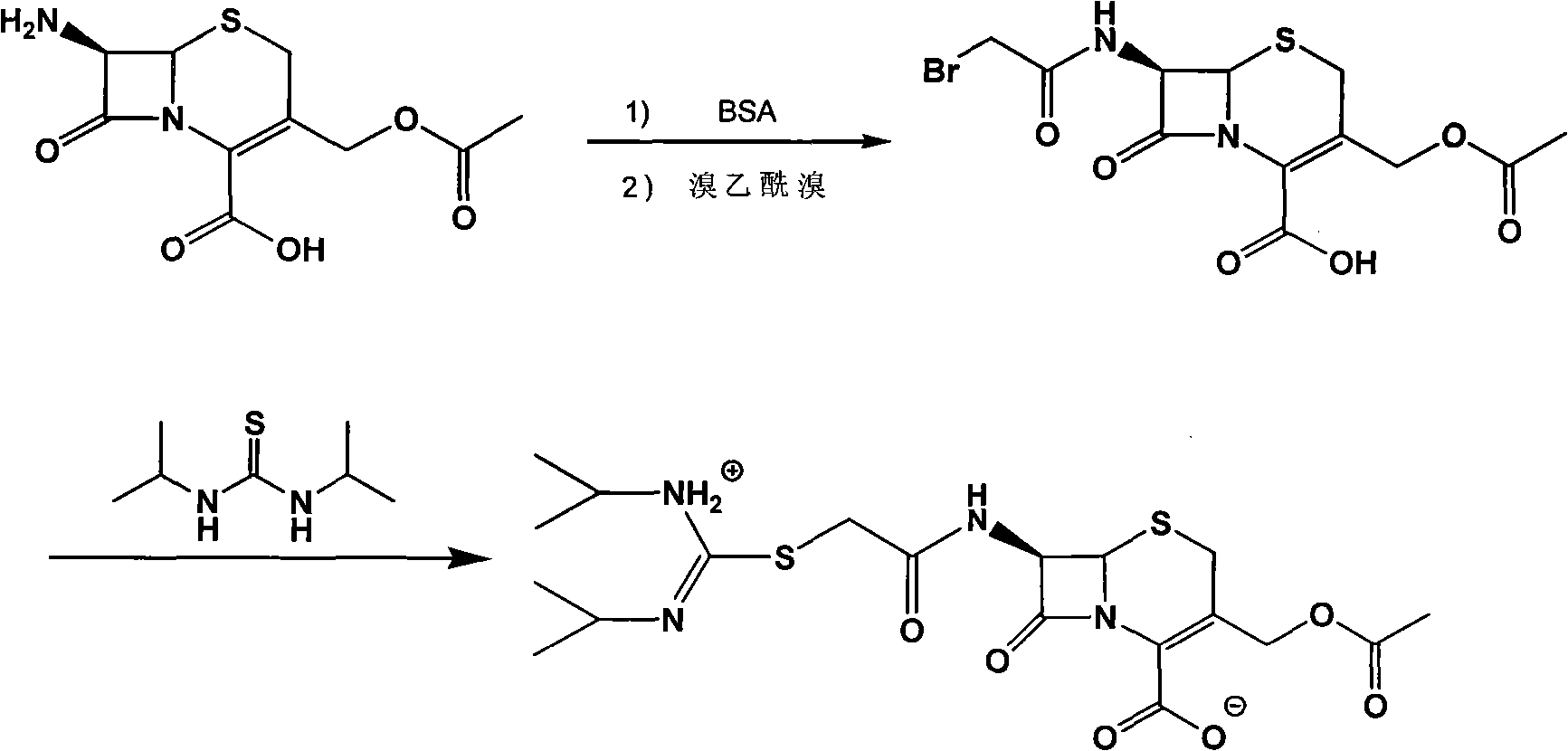
[0028] Example 1 Synthesis of Cefathiamidine Acid
[0029] Add 50 ml of dichloromethane, 12 g (0.044 mol) of 7-ACA, and 11.2 g of BSA to a 250 ml round bottom flask, and stir at room temperature for 2 hours to dissolve. Then, 8.82g (0.044mol) of bromoacetyl bromide was slowly added dropwise at 0°C. After the dropwise addition, the reaction temperature can slowly rise to room temperature and react for 2h. 100ml of distilled water was added to the reaction solution, a large amount of solids precipitated, stirring was continued for half an hour, suction filtration, the filter cake was washed with water, and vacuum dried to obtain 15.56g of cefathiamidine acid. The yield was 90.2%.
Embodiment 2
[0030] Example 2 Synthesis of crude cefathiamidine
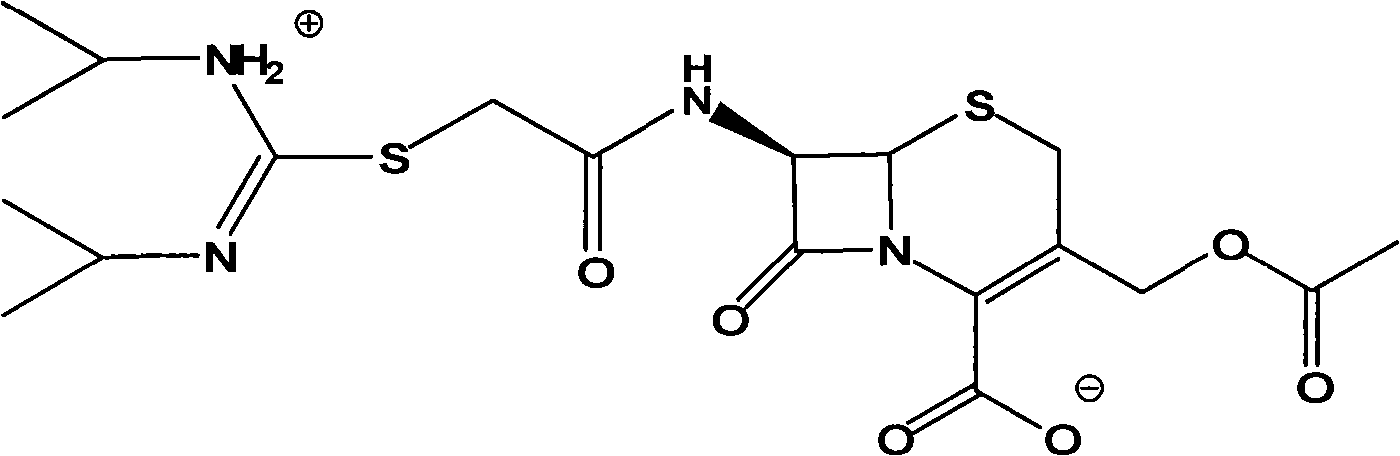
[0031] Add 3.93g cefathiamidine acid, 40ml dichloromethane, 1.3ml triethylamine into a 150ml round bottom flask, the solution becomes clear, add 1.6g N,N'-diisopropylthiourea, stir at room temperature for 3 hours, TLC The detection reaction is complete. Slowly add 50ml acetone dropwise, a solid precipitates, continue to stir for 2h, and let it stand for half an hour. Filter with suction and wash with acetone. After vacuum drying, 4.45 g of crude cefathiamidine was obtained. The yield was 94.1%.
Embodiment 3
[0032] Example 3 Refined crude cefathiamidine
[0033] Add 5g of crude cefathiamidine to a 100ml round bottom flask, dissolve it with 5ml of distilled water, adjust the pH to 5.5 with hydrochloric acid, add a certain amount of acetone until the solution becomes turbid, control the stirring speed, continue to slowly add 50ml of acetone, crystallize for 3h, filter with suction, and wash , Dried under vacuum to obtain 4.6g of cefathiamidine. The purity is 98.48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com