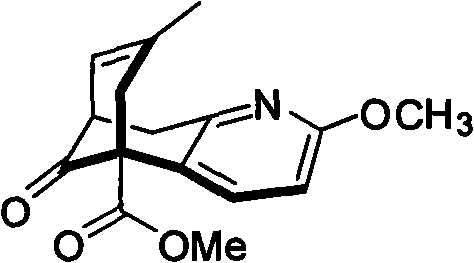
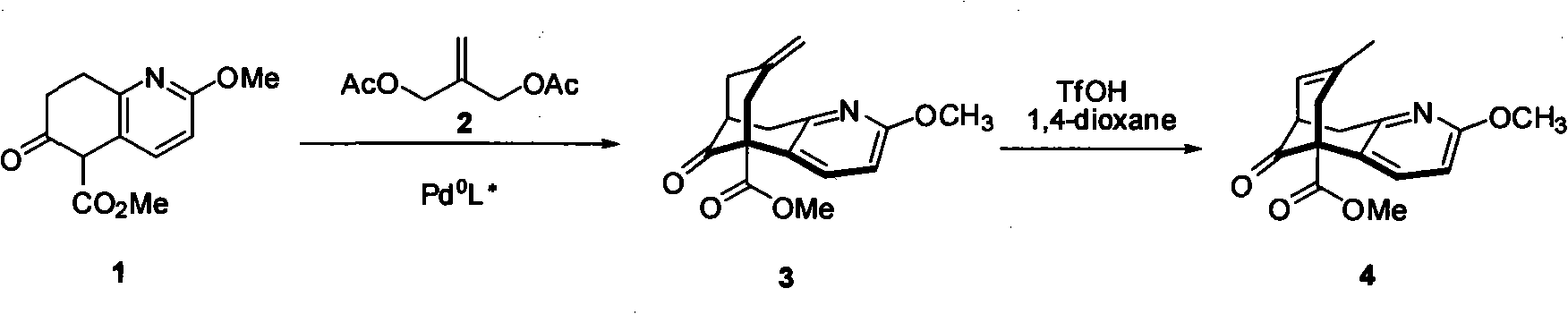
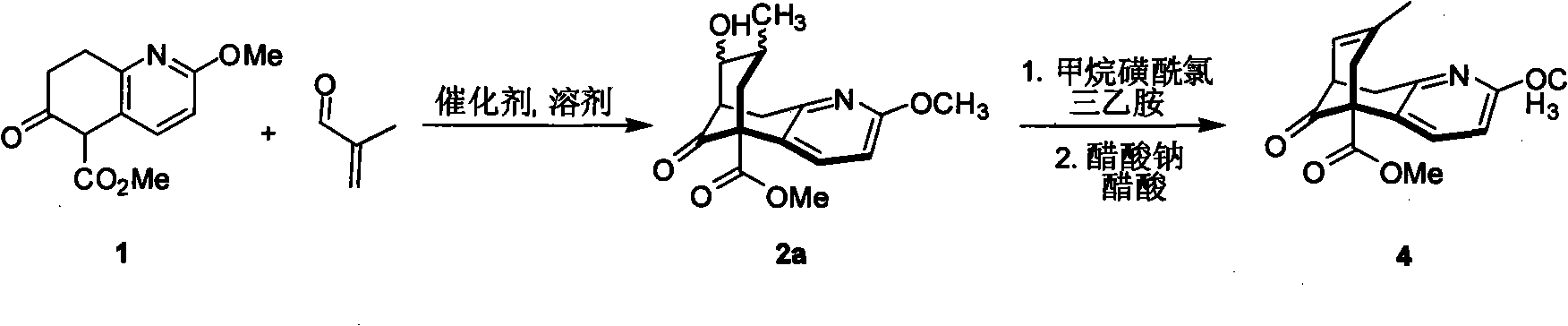
Asymmetric synthetic method of (-)-huperzine key intermediate
A synthesis method and intermediate technology are applied in the field of asymmetric synthesis for synthesizing huperzine key intermediates, which can solve problems such as uneconomical, and achieve the effects of high yield and simple post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 7,8,9,10-tetrahydro-8-hydroxy-2-methoxy-7-methyl-11-oxo-5,9-methylenecyclooctane[b]pyridine-5(6H )-methyl carboxylate
[0038] Methyl 5,6,7,8-tetrahydro-2-methyl-6-oxo-5-quinolinecarboxylate (940mg, 4mmol), 2-methyl-acrolein (140mg, 8mmol), catalyst (316mg, 1.2mmmol), additive NaOAc (336mg, 4mmol), added in THF, stirred, refluxed for 4 to 5 days, the reaction solution was cooled, washed three times with saturated sodium bicarbonate solution, washed twice with saturated sodium chloride, The crude product was subjected to column chromatography to obtain 2a (660 mg) and 2b (340 mg), wherein 2b was subjected to sodium hydroxide / dichloromethane at room temperature for 6 hours, and the crude product was subjected to column chromatography to obtain 2a (306 mg). Or use the enantiomer of the optically active catalyst to do the opposite configuration of 2a. The main configuration of 2a 1 HNMR (CDCl 3): 7.06(d, 1H, J=7.1Hz), 6.62(d, 1H, J=7.1Hz), 3.91(s, 3H), 3.81(s, 3H), 3...
Embodiment 2
[0043] 7,8,9,10-tetrahydro-8-hydroxy-2-methoxy-7-methyl-11-oxo-5,9-methylenecyclooctane[b]pyridine-5(6H )-methyl carboxylate
[0044] Methyl 5,6,7,8-tetrahydro-2-methyl-6-oxo-5-quinolinecarboxylate (940mg, 4mmol), 2-methyl-acrolein (140mg, 8mmol), catalyst (388mg, 1.2mmmol), additive trifluoroacetic acid (456mg, 4mmol), added to tetrahydrofuran, stirred, refluxed for 4 to 5 days, the reaction solution was cooled, washed three times with saturated sodium bicarbonate solution, washed two times with saturated sodium chloride The second time, the crude product was subjected to column chromatography to obtain 2a (620 mg) and 2b (320 mg), wherein 2b was subjected to sodium hydroxide / dichloromethane at room temperature for 6 hours, and the crude product was subjected to column chromatography to obtain 2a (290 mg). Or use the enantiomer of the optically active catalyst to do the opposite configuration of 2a. The main configuration of 2a 1 HNMR (CDCl 3 ): 7.06(d, 1H, J=7.1Hz), 6...
Embodiment 3
[0049] 7,8,9,10-tetrahydro-8-hydroxy-2-methoxy-7-phenyl-11-oxo-5,9-methylenecyclooctane[b]pyridine-5(6H )-methyl carboxylate
[0050] Methyl 5,6,7,8-tetrahydro-2-methyl-6-oxo-5-quinolinecarboxylate (940mg, 4mmol), 2-phenyl-propenal (140mg, 8mmol), catalyst (328mg, 1.3mmmol), additive sodium methoxide (189mg, 3.5mmol), added to tetrahydrofuran, stirred, refluxed for 4 to 5 days, the reaction solution was cooled, washed with saturated sodium bicarbonate solution three times, washed with saturated sodium chloride for two Once, the crude product was subjected to column chromatography to obtain 2a-1 (600mg) and 2b-1 (300mg), wherein 2b-1 was subjected to sodium hydroxide / methylene chloride at room temperature for 6 hours, and the crude product was obtained by column chromatography to obtain 2a-1 ( 280mg). Or use the enantiomer of the optically active catalyst to do the opposite configuration of 2a-1.
[0051] (-)-(5S,9R)-9,10-dihydro-2-methoxy-7-phenyl-11-oxo-5,9-methylenecyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com