Method for combined production of ammonia and basic calcium chloride by decomposing ammonium chloride
A technology of ammonium chloride and calcium chloride, applied in the direction of calcium/strontium/barium chloride, ammonia preparation/separation, calcium/strontium/barium halide, etc., which can solve transportation difficulties, slow reaction speed, and large heat consumption and other problems, to achieve the effect of simple process, high conversion rate and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
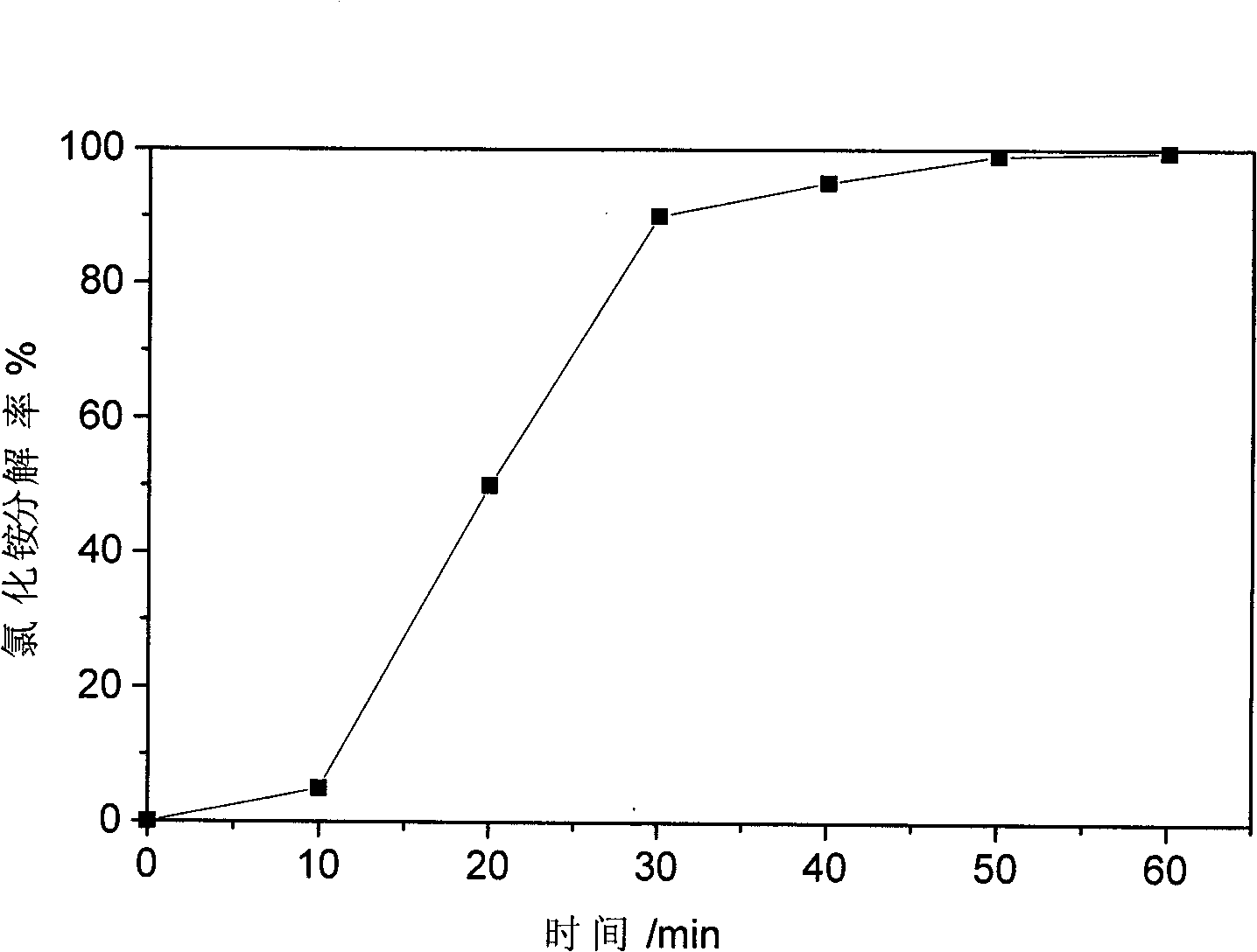
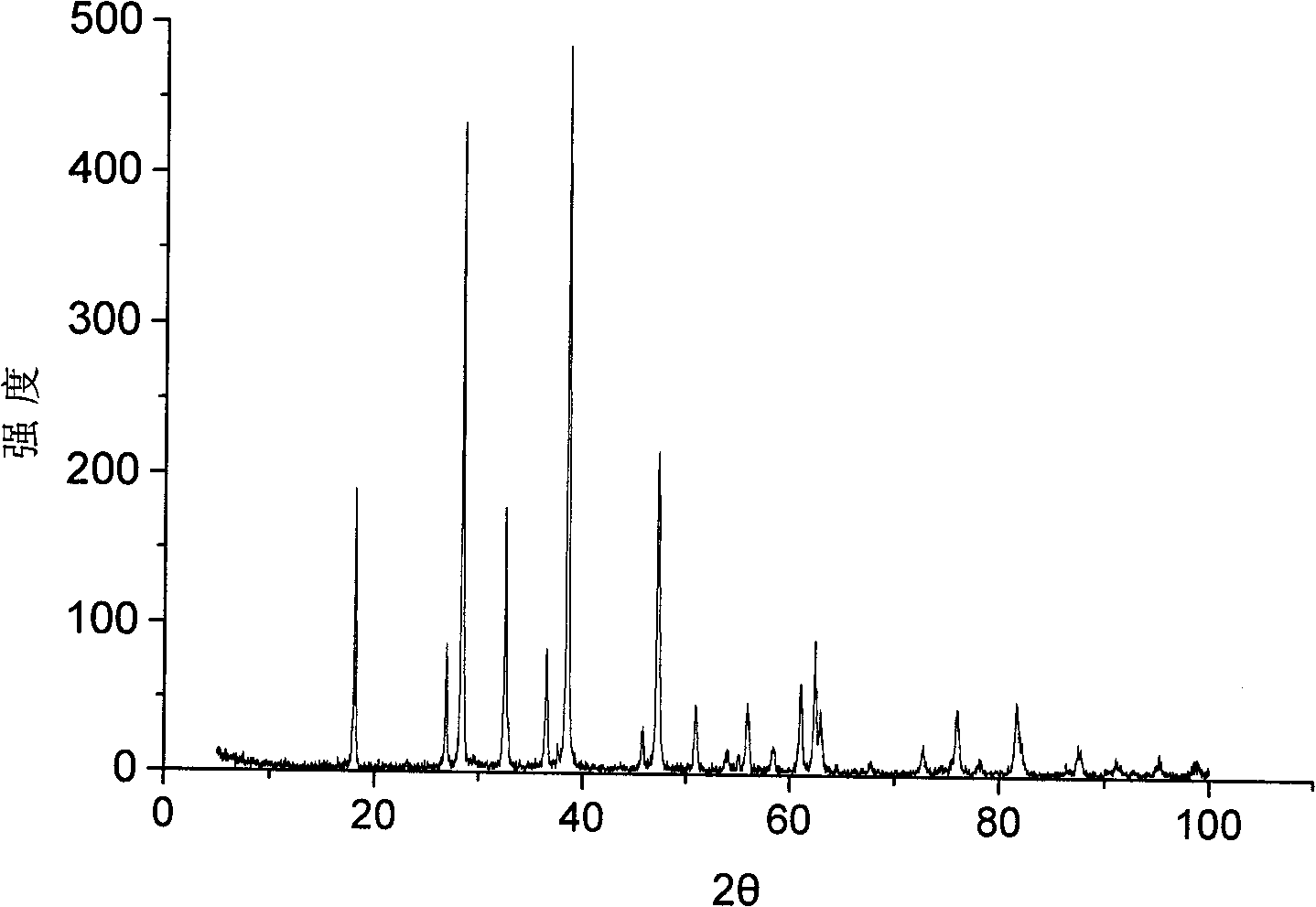
[0027] Put 56g of calcium oxide with a particle size of 5mm in diameter and 53g of ammonium chloride into the pulverizer at the same time. The pulverizer model is SL-30. The pulverizer is turned on and the pulverization time is 60 minutes. Such as figure 1 shown. As can be seen from the figure, as the crushing time increases, the decomposition rate of ammonium chloride increases, and when the crushing time reaches 60 minutes, the decomposition rate of ammonium chloride reaches 99.5%. figure 2 It is the X-ray powder diffraction pattern of the solid product basic calcium chloride. Compared with the standard spectrum, it can be seen that the reaction between ammonium chloride and calcium oxide is basically completed, and it is completely converted into basic calcium chloride.
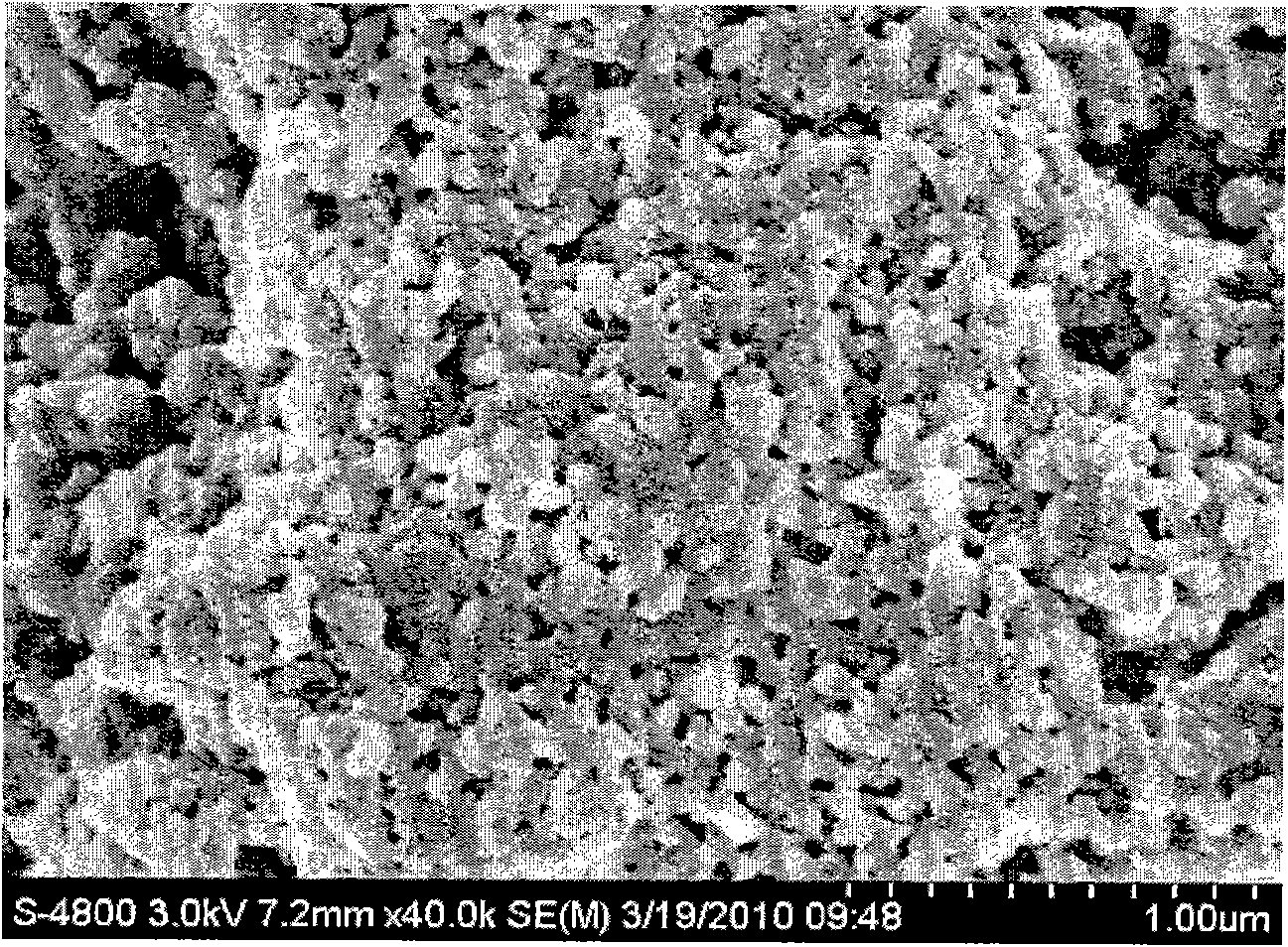
[0028] image 3 It is a scanning electron micrograph of the product basic calcium chloride. It can be seen from the figure that its particle size is about 100 nanometers.
Embodiment 2
[0030] Put 100g of calcium hydroxide and 71.62g of ammonium chloride with a particle size of 10mm in diameter into the ball mill at the same time, turn on the ball mill, and the ball milling time is 80 minutes. The ammonium chloride decomposition rate varies with the pulverization time as follows: Figure 4 shown. As can be seen from the figure, as the crushing time increases, the decomposition rate of ammonium chloride increases, and when the crushing time reaches 80 minutes, the decomposition rate of ammonium chloride reaches 99%. Figure 5 It is the X-ray powder diffraction pattern of the solid product basic calcium chloride. Compared with the standard spectrogram, it can be seen that the reaction between ammonium chloride and calcium hydroxide is basically complete, and it is completely converted into basic calcium chloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com