Process for producing nanoparticles
A technology of metal carboxylates and mixtures, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of insufficient particle size control, slow particle growth rate, high agglomeration rate, etc., to achieve simple method and particle agglomeration Minimized, simple cost-effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0057] Objects and advantages of this invention are further illustrated by the following examples, but the particular materials and amounts thereof recited in these examples, as well as other conditions and details, should not be construed to unduly limit this invention.
[0058] These examples are for illustration purposes only and are not intended to limit the scope of the appended claims.
[0059] All parts, percentages, ratios, etc. in the examples and in the remainder of the specification are by weight unless otherwise indicated. Solvents and other reagents used were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO) unless otherwise indicated.
[0060] Primary particle size determination
[0061] The ultraviolet (UV) visible (Vis) spectrometer used to monitor the primary particle size is PerkinElmer TMLambda 35 instrument (available from PerkinElmer Life and Analytical Sciences, Wellesley, MA) with a 1 cm path length UV grade quartz sample cell or cuvette. ...
example 1-4 and comparative example 1
[0080] Zinc lactate (Pfaltz & Bauer, Waterbury, CT) was dried overnight in a vacuum oven at 100°C. Zinc lactate was subjected to thermogravimetric analysis (TGA) before and after drying. The temperature of the thermogravimetric analyzer was raised at a rate of 20°C per minute to a temperature of 120°C and held at this temperature for 20 minutes. The zinc lactate contained 15.7% by weight of water before drying in a vacuum oven. After drying, the zinc lactate contained 2.4% by weight of water.
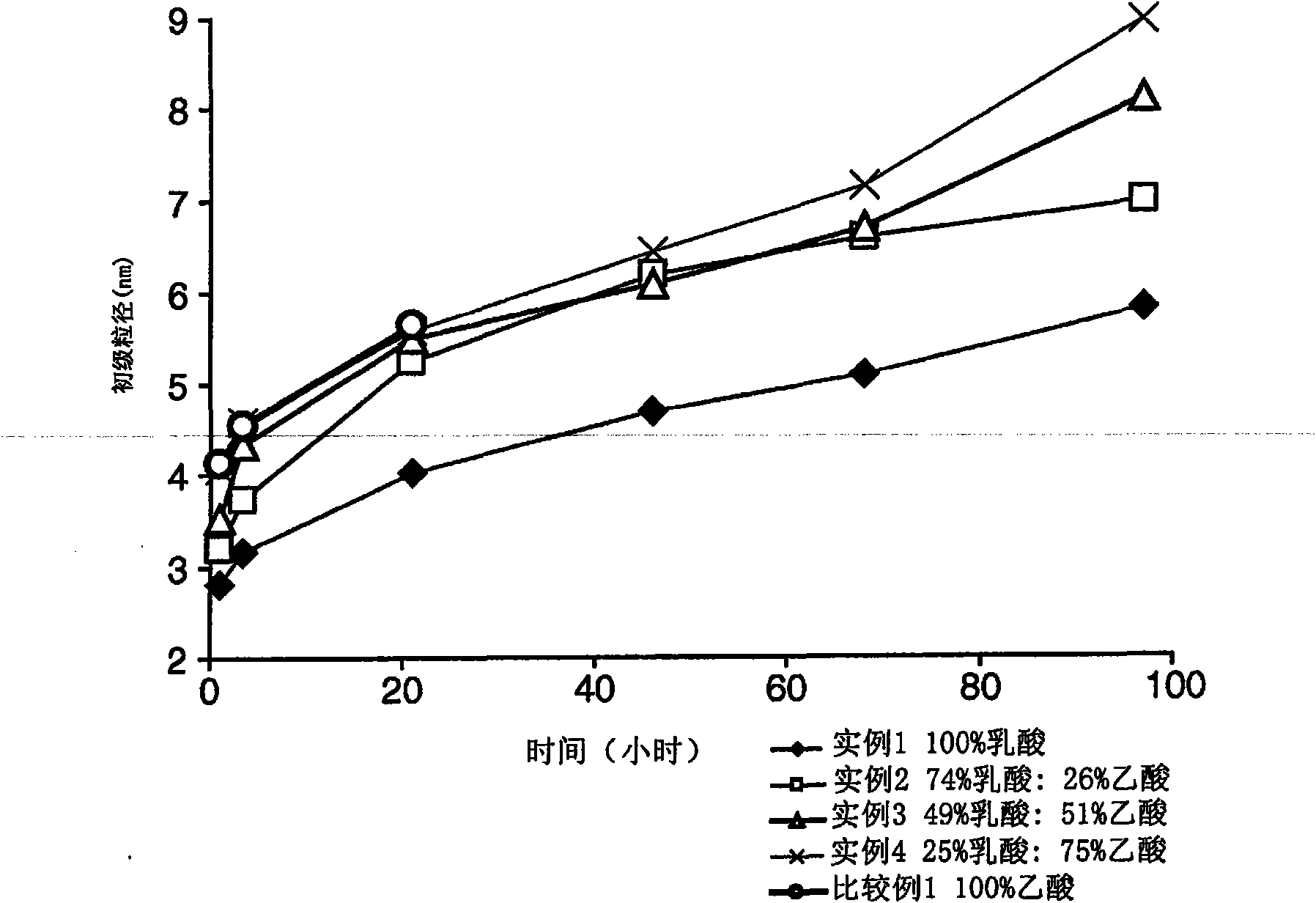
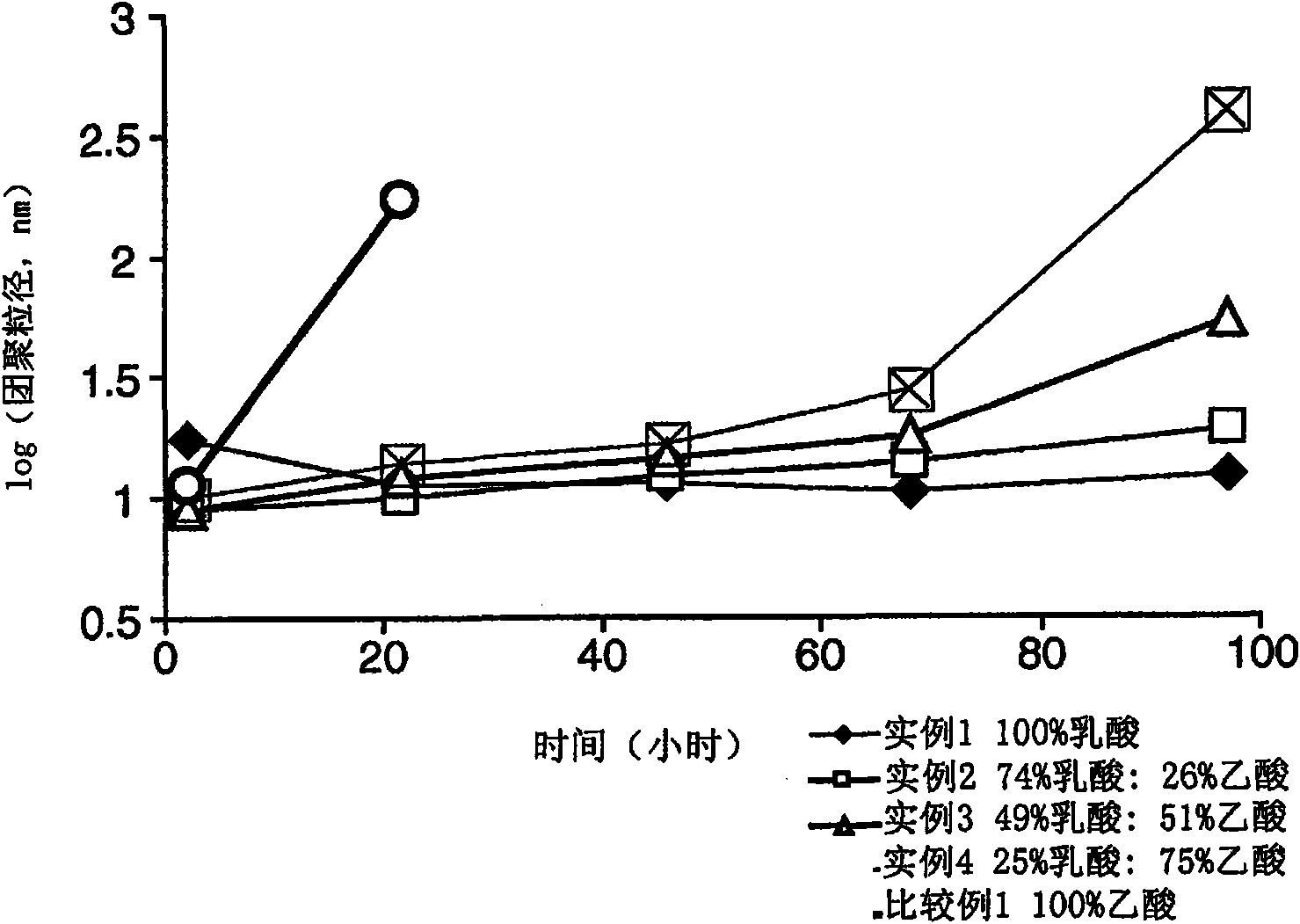
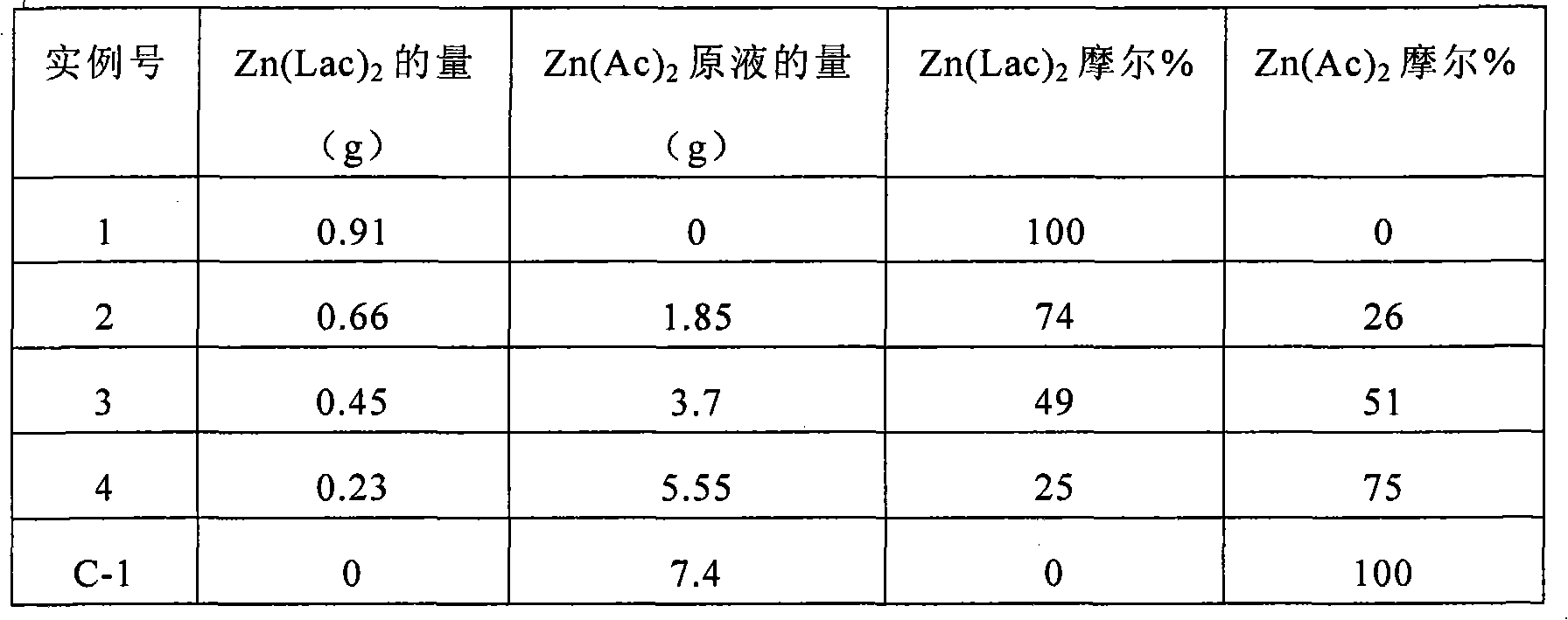
[0081] Using different proportions of zinc lactate (Zn(Lac) 2 ) and zinc acetate (Zn(Ac) 2 ) to synthesize zinc oxide. A 0.52 mmol / g Zn(Ac) 2 stock solution. Different amounts of Zn(Lac) 2 and DMSO were added to each of this stock solution to prepare a series of samples, as shown in Table 1 below.
[0082] Table 1.
[0083]
[0084] Each sample was placed in a 40 mL vial, and 20.3 g of DMSO and a magnetic stir bar were added to each vial. Each vial was then placed in an ...
Embodiment 5
[0087] Zinc lactate (Pfaltz & Bauer, Waterbury, CT) was vacuum dried at 100° C. overnight essentially as described above. TGA was performed essentially as described above and the results showed that the zinc lactate contained 4.6% by weight of water after drying.
[0088] DMSO (200 g) was placed in a 1 liter 3 neck round bottom flask. Zinc acetate (28.44 g, 0.155 moles, Alfa Aesar, Ward Hill, MA) and vacuum-dried zinc lactate (37.74 g, 0.155 moles) were added as powders to the flask via a powder addition funnel under mechanical stirring. DMSO (41.9 g) was used to rinse residual zinc acetate or zinc lactate from the powder addition funnel into the round bottom flask. The flask was placed in a silicone oil bath set at 90°C. After the powder had dissolved, a solution of 25% tetramethylammonium hydroxide in methanol (192.1 g, 0.527 moles, Alfa Aesar, Ward Hill, MA) was added to the flask in a steady stream over 15 minutes via a separatory funnel.
[0089]The size of the resulti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com