Animal medicine inclusion compound, preparation method and application thereof
A technology of inclusion compounds and veterinary drugs, applied to veterinary drug inclusion compounds and preparation thereof, and the application field of veterinary drug inclusion compounds in the preparation of veterinary drugs, can solve the problem of failure to disclose inclusion compounds on solubility and stability, limited convenience and Economical, poor animal palatability, etc., to achieve the effects of improving hygroscopicity, reducing toxic and side effects, and facilitating application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
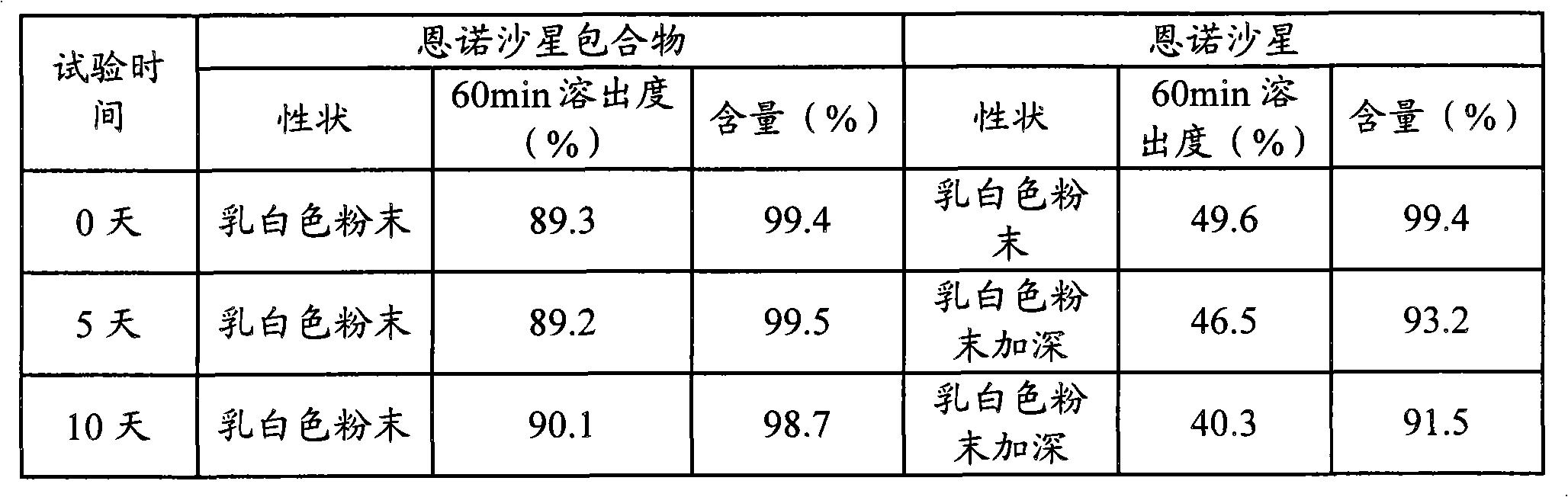
[0031] Example 1 Preparation and Properties of Enrofloxacin Inclusion Compound
[0032] 1. Preparation of enrofloxacin inclusion complex
[0033] Weigh 14.40g (0.04mol) of enrofloxacin and 61.64g (0.04mol) of hydroxypropyl-β-cyclodextrin, place hydroxypropyl-β-cyclodextrin in a beaker, add 100ml of distilled water, and Prepare a saturated solution of hydroxypropyl-β-cyclodextrin on a water bath at ℃; take another enrofloxacin, add it to saturated hydroxypropyl-β-cyclodextrin, stir on a water bath at 70℃ for 30 minutes, and place it in a freeze dryer immediately Pre-freeze at -40°C for 12 hours, freeze-dry at -30°C, pass through a 100-mesh sieve to obtain enrofloxacin inclusion compound.
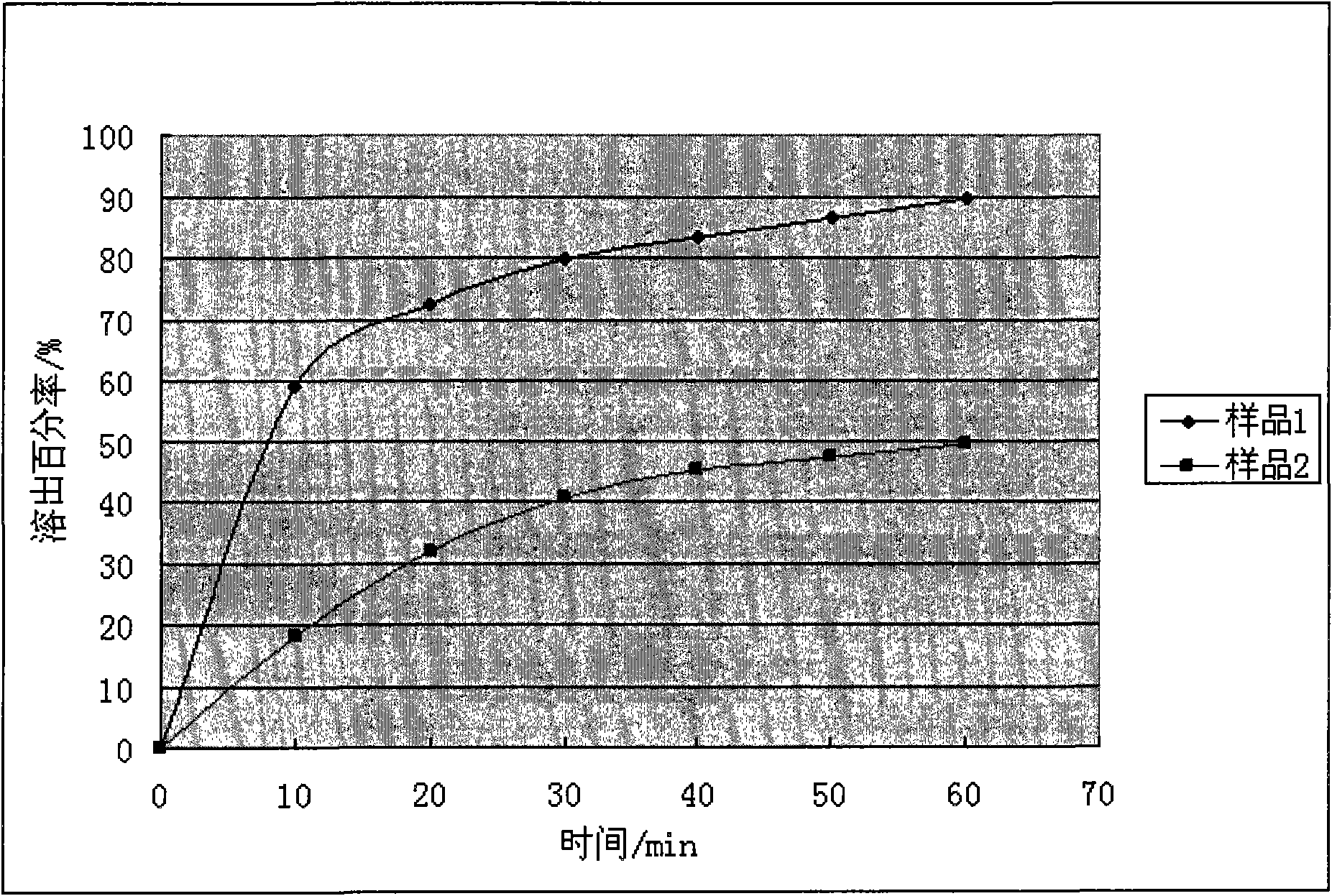
[0034] 2. Determination of dissolution rate of enrofloxacin inclusion compound
[0035] 1) Determination of Enrofloxacin Content
[0036] Chromatographic conditions: Octadecylsilane bonded silica gel is used as filler; column temperature is 40°C; detection wavelength is 278nm; theoretical ...
Embodiment 2
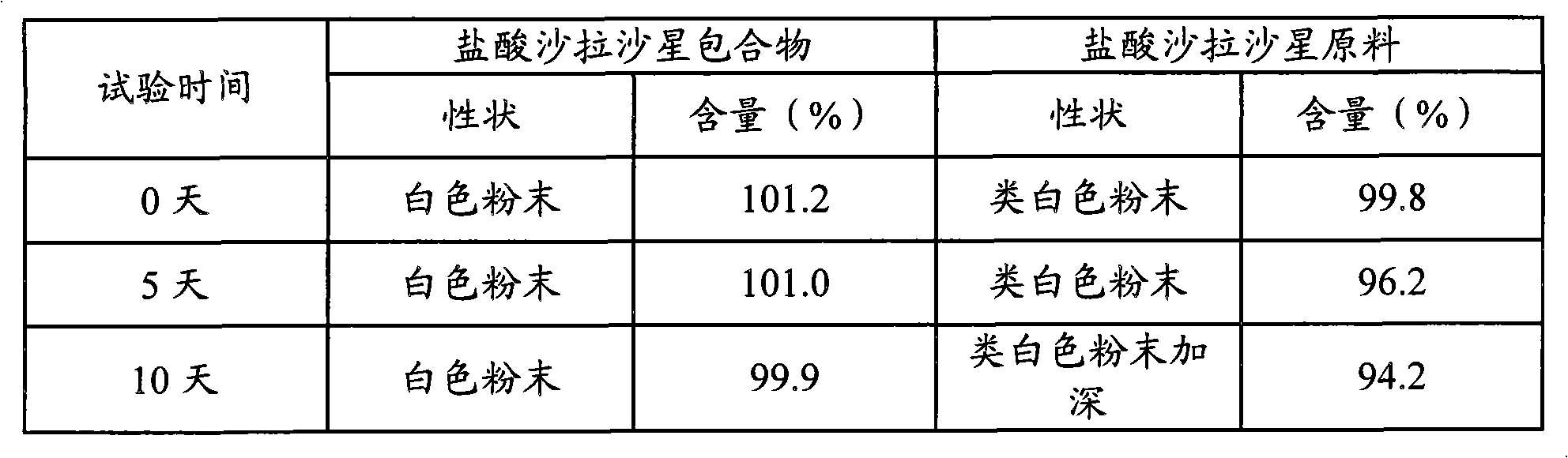
[0057] Example 2 Preparation of Sarafloxacin Hydrochloride Inclusion Compound and 1% W / V Sarafloxacin Hydrochloride Solution
[0058] 1. Sarafloxacin hydrochloride inclusion compound
[0059] Weigh 16.91 g (0.04 mol) of sarafloxacin hydrochloride and 61.64 g (0.04 mol) of hydroxypropyl-β-cyclodextrin, and prepare according to the method described in Example 1.
[0060] The solubility of sarafloxacin hydrochloride inclusion compound is measured with reference to the method of Example 1. The solubility of sarafloxacin hydrochloride inclusion compound is 1.41 mg / ml, and the solubility of sarafloxacin hydrochloride raw material is 0.067 mg / ml, that is, sarafloxacin hydrochloride inclusion compound The solubility is 21.0 times that of sarafloxacin hydrochloride raw material.
[0061] According to the technical guidelines for the stability research of veterinary chemical drugs, high-temperature test research was carried out on the inclusion compound of sarafloxacin hydrochloride. T...
Embodiment 3
[0070] Example 3 Ciprofloxacin Lactate Inclusion Compound and Stability Determination
[0071] Ciprofloxacin lactate has hygroscopicity, and the clathrate technology is used to clathrate ciprofloxacin lactate, which can prevent ciprofloxacin lactate from attracting moisture during storage.
[0072] 1. Preparation of inclusion compound of ciprofloxacin lactate: Weigh 16.92g (0.04mol) of ciprofloxacin lactate and 58.60g (0.04mol) of 2,6-dimethyl-β-cyclodextrin, according to Example 1 prepared by method.
[0073] 2. Stability test of ciprofloxacin lactate inclusion compound
[0074] Carry out high-humidity test research on the ciprofloxacin lactate inclusion compound according to the technical guidelines for the stability research of veterinary chemical drugs. Place it under the condition of humidity 90%±5% for 10 days, take samples on the 5th and 10th days, and compare with the properties, moisture absorption weight gain, and content detection results on the 0th day; the raw m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com