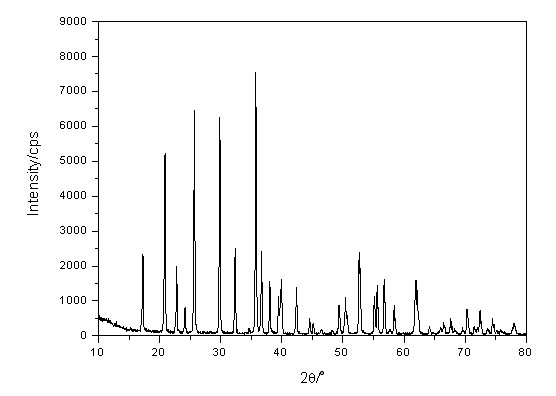
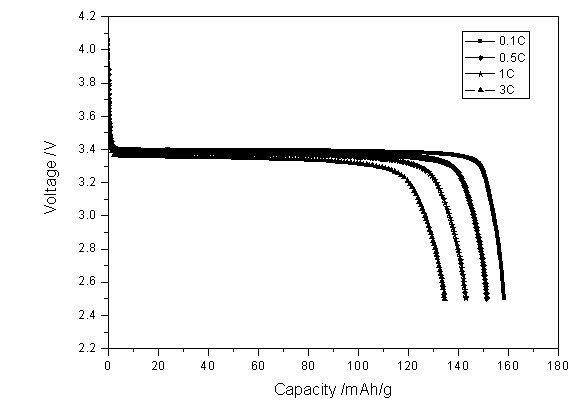
Method for synthesizing lithium iron phosphate anode material at low cost
A technology of lithium iron phosphate and positive electrode materials, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., and can solve problems such as difficult to mix the stoichiometric ratio of the three raw materials uniformly, difficulty in lithium iron phosphate, and low tap density , to achieve the effect of improving electrochemical performance, reducing primary particle size, and small primary particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Take by weighing 12000g deionized water and place in the acid-proof reaction kettle, under the situation of stirring, slowly pour into 5000g H 3 PO 4 (mass fraction 85%), diluted H 3 PO 4 The mass fraction is 25%. Weigh 1820g LiOH·H 2 O was slowly added to the above solution and stirred for 30 min to obtain a colorless solution. Transfer all the colorless solution to the stirring ball mill, add 3469g Fe 2 o 3 , 170g polyvinylpyrrolidone (PVP), 374g acetylene black. Grind in a stirring ball mill for 3 hours at 300 rpm to obtain a brown-red suspension, namely the precursor slurry. The precursor slurry was transported to the atomizing disc of the spray dryer at a flow rate of 0.6L / min by a metering pump under constant stirring, the inlet temperature was set at 300°C, and the outlet temperature was set at 120°C. Collector can get brownish red precursor powder. Put the precursor powder into a sagger, place it in a rotary furnace with high-purity nitrogen protection ...
Embodiment 2
[0031] Take by weighing 10000g deionized water and place it in an acid-resistant reaction kettle, and slowly pour 5000g H 3 PO 4 (mass fraction 85%), diluted H 3 PO 4 The quality fraction of is 28.3%. Weigh 1820g LiOH·H 2 O was slowly added to the above solution and stirred for 30 min to obtain a colorless solution. Transfer all the colorless solution to the stirring ball mill, add 3347g Fe 3 o 4 , 204g soluble starch, 272g superconducting carbon black. Grinding with a stirring ball mill at 200 rpm for 4 hours to obtain a black suspension, namely the precursor slurry. The precursor slurry was transported to the atomizing disc of the spray dryer at a flow rate of 0.4L / min by a metering pump under constant stirring, the inlet temperature was set at 280°C, and the outlet temperature was set at 110°C. The collector yields a black precursor powder. Put the precursor powder into a sagger, place it in a rotary furnace with high-purity nitrogen protection (gas flow rate is 2L...
Embodiment 3
[0033] Take by weighing 11000g deionized water and place in the acid-resistant reaction kettle, under the situation of stirring, slowly pour into 5000g H 3 PO 4 (mass fraction 85%), diluted H 3 PO 4 The quality fraction of is 26.6%. Weigh 1602g Li 2 CO 3 Slowly added to the above solution and stirred for 30min to obtain a colorless solution. Transfer all the colorless solution to the stirring ball mill, add 3469g Fe 2 o 3 , 136g polyvinyl alcohol (PVA), 374g acetylene black. Grind in a stirring ball mill at 300 rpm for 3 hours to obtain a brownish-red suspension, namely the precursor slurry. The precursor slurry was transported to the atomizing disc of the spray dryer at a flow rate of 0.4L / min by a metering pump under constant stirring, the inlet temperature was set at 320°C, and the outlet temperature was set at 135°C. Collector can get brownish red precursor powder. The precursor powder was put into a sagger, placed in a rotary furnace with high-purity nitrogen pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| capacitance | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com