Method for synthesizing gamma lactone
A synthesis method and lactone technology, applied in the direction of organic chemistry, can solve the problems of reduced reaction efficiency, complicated reaction device, and influence on product purity, and achieve the effect of improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1 (gamma undecalactone)
[0016] a ingredient
[0017] 172g (2.0mol) of methyl acrylate; 2000g (15.4mol) of octanol; 30g (0.2mol) of di-tert-butyl peroxide; 1.7g of SL-90 polymerization inhibitor was added to stirring pot 1, and the temperature dropped to -5°C , stirring for 10 minutes;
[0018] b reaction
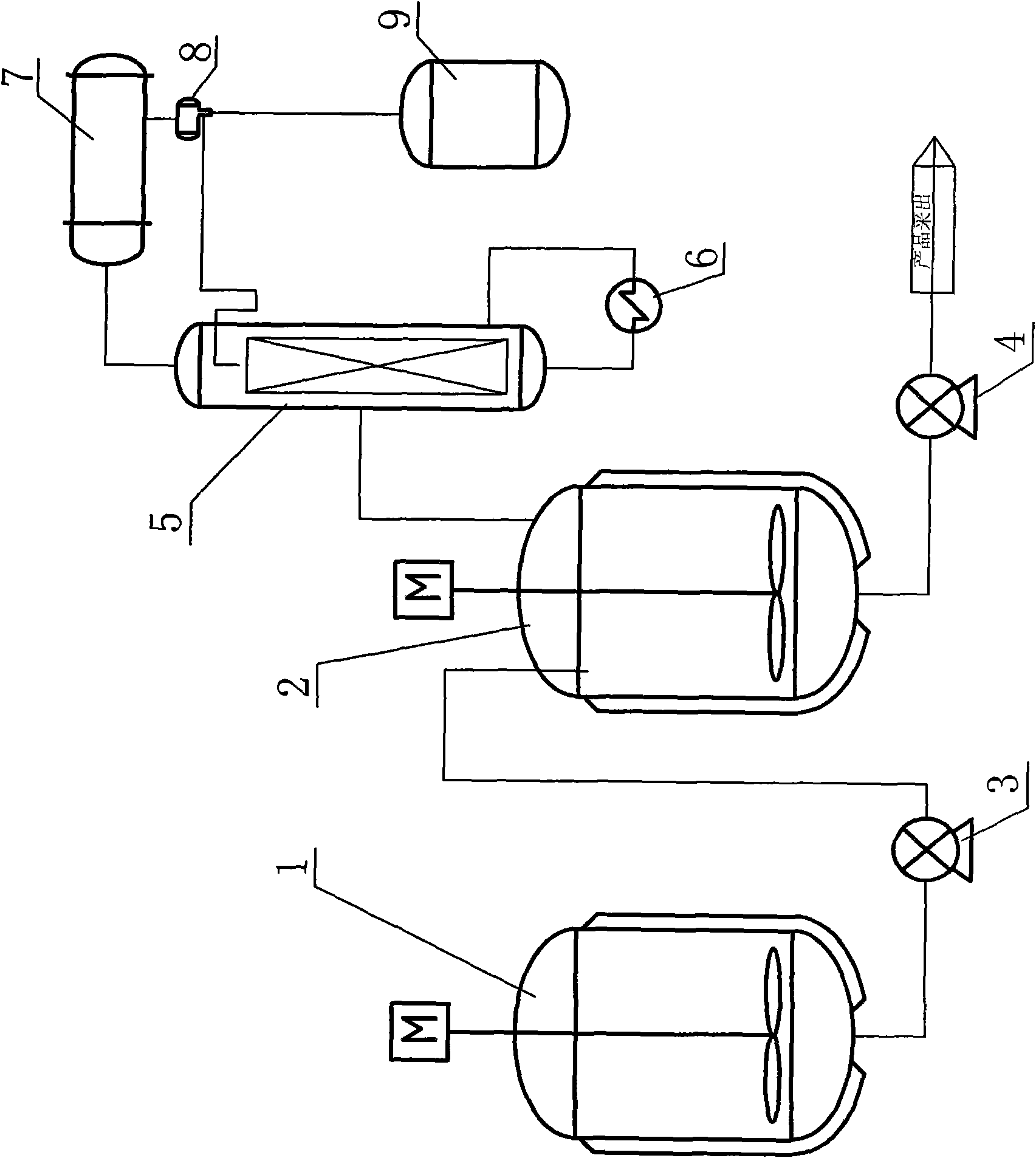
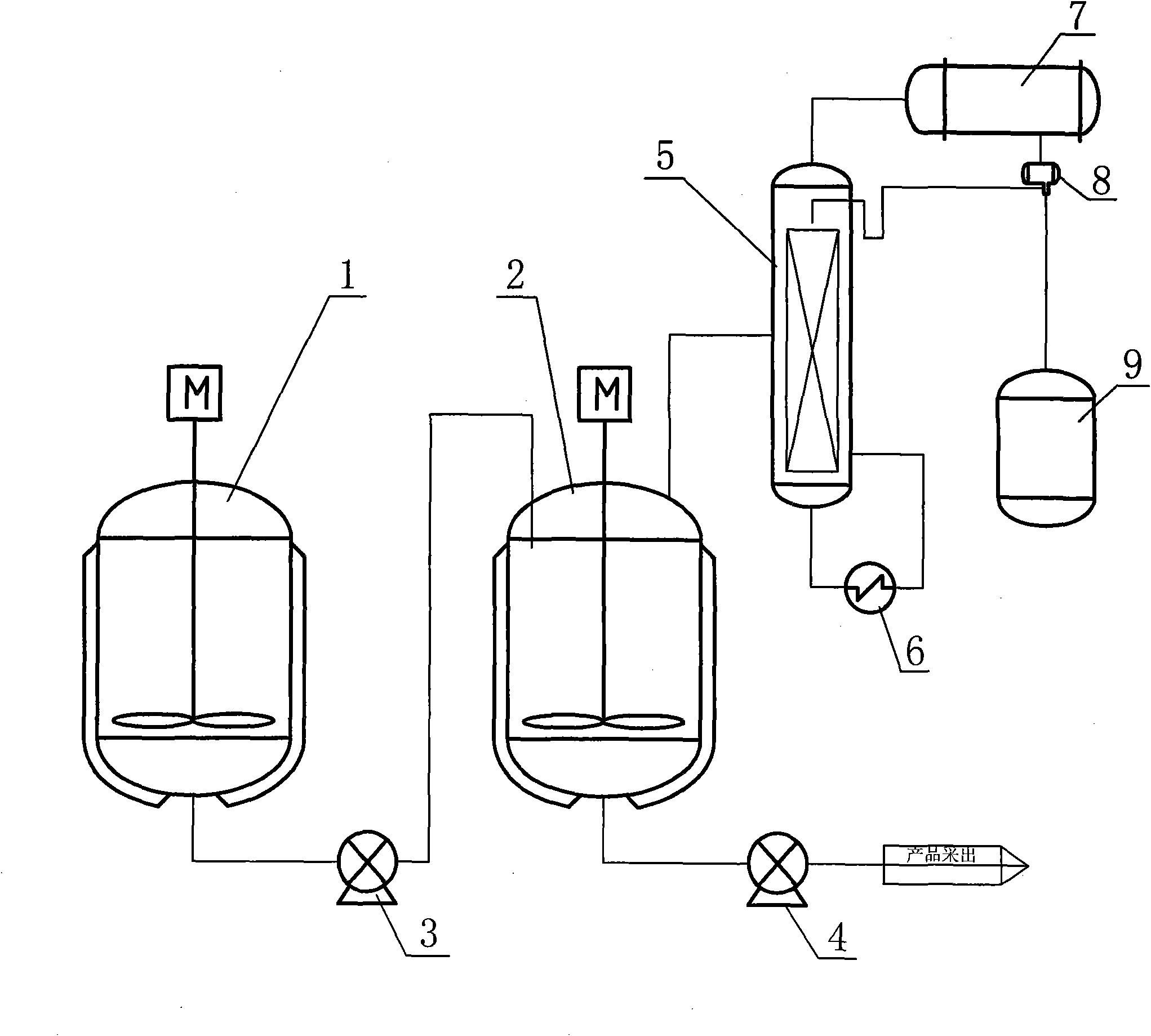
[0019] The above-mentioned ingredients are continuously added into the 1L autoclave 2 through the pump 3 through the feeding device, and the flow rate is controlled at 160g / hour; the nitrogen is pressurized to 0.25Mpa, and the temperature is raised and controlled at 190°C; Product methanol, tert-butanol and other by-products; unreacted octanol is recovered to the reflux tank 8 at the bottom of the tower; the reaction product is continuously extracted from the pump 4, and the flow rate of the extracted reactant is controlled to maintain the liquid level of the reactor; after 10 hours, the reaction The content of the crude product reaches and stabilizes ...
Embodiment 2
[0021] Embodiment 2 (gamma nonalactone)
[0022] a ingredient
[0023] 200g of methyl acrylate; 1900g of hexanol; 40g of di-tert-butyl peroxide; 1.7g of SL-90 polymerization inhibitor was added to the stirring pot 1, the temperature dropped to -5°C, and stirred for 10 minutes;
[0024] b reaction
[0025] The above-mentioned ingredients are continuously added into the 1L autoclave 2 through the pump 3 through the feeding device, and the flow rate is controlled at 160g / hour; the nitrogen is pressurized to 0.25Mpa, and the temperature is raised and controlled at 190°C; Product methanol, tert-butanol and other by-products; unreacted hexanol is recovered to the reflux tank 8 at the bottom of the tower; the reaction product is continuously extracted from the pump 4, and the flow rate of the extracted reactant is controlled to maintain the liquid level of the reactor; after 10 hours, the reaction The content of the crude product reaches and stabilizes at 20%. After high-vacuum pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com