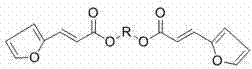
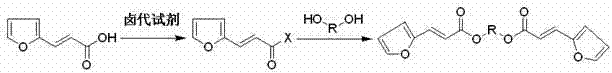
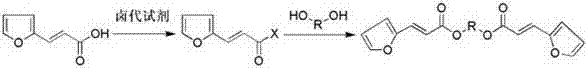
Mono-pentose or hexose-(E)-3-(furan-2-yl) acrylic acid diester compound and preparation method and use thereof
A technology of diester acrylate and six-carbon monosaccharide, which is applied in the field of tobacco, can solve the problems of unsatisfactory moisture content and smoking comfort of finished cigarettes, enhanced dryness and irritation, and greater influence of tobacco. Conducive to the effects of smoking safety, comfortable and harmonious aroma, and easy promotion and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of 3,6-di-[(2E)-3-(furan-2-yl)-2-acryloyl]-D-glucopyranosyl ester (compound I):
[0035] Add 16.58 g (0.12 mol) of (E)-3-(furan-2-yl)acrylic acid, 28.56 g (0.24 mol) of thionyl chloride and 200 ml of chloroform in sequence in the reaction flask, and heat up to 50-55°C to stir the reaction After 3 h, after the reaction, the solvent and excess thionyl chloride were distilled off under reduced pressure, the residue was dissolved in 100 ml of dichloromethane, and 9.7 g of D-glucosylmethyl glycoside which had been cooled to -5 ~ 0 °C was added dropwise (0.05 mol), triethylamine 20.2g (0.20 mol) andN , N - In 100 ml of dimethylformamide mixed solution, stirred at 15-30°C for 20 h, after the reaction, the solid was precipitated by filtration, the filtrate was evaporated under reduced pressure to remove the solvent, the residue was dissolved in 200 ml of dichloromethane, and 10% Hydrochloric acid aqueous solution 40 ml, stirred at room temperature for 2 h, the orga...
Embodiment 2
[0037] Preparation of 2,3-di-[(2E)-3-(furan-2-yl)-2-acryloyl]-D-glucopyranosyl ester (compound II):
[0038] The operation process is the same as in Example 1, except that thionyl chloride is replaced with phosphorus trichloride, and triethylamine is replaced with pyridine, N , N -Dimethylformamide is replaced by ethyl acetate, and D-glucose methyl glycoside is replaced by 4,6-O-isopropylidene-D-glucose methyl glycoside to obtain 2,3-di-[(2E)- 3-(Furan-2-yl)-2-acryloyl]-D-glucopyranosyl ester, yield 64.5%; HR-TOFMS (+Q) m / z : 421.1156 ( [C 20 h 20 o 10 +H] + Calculated value: 421.1153 ).
Embodiment 3
[0040] Preparation of 2,3-bis-[(2E)-3-(furan-2-yl)-2-acryloyl]-D-galactopyranose ester (compound III):
[0041] Operation process is the same as embodiment 1, just uses thionyl chloride a -Chloro- N,N,2 -Trimethyl allylamine is replaced, triethylamine is replaced by pyridine, N , N -Dimethylformamide is replaced by tetrahydrofuran, and D-glucosylmethyl glycoside is replaced by 4,6-O-isopropylidene-D-galactosylmethyl glycoside to obtain 2,3-di-[(2E)-3 -(furan-2-yl)-2-acryloyl]-D-galactopyranose ester, yield 71.8%; HR-TOFMS (+Q) m / z : 421.1145 ( [C 20 h 20 o 10 +H] + Calculated value: 421.1153 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com