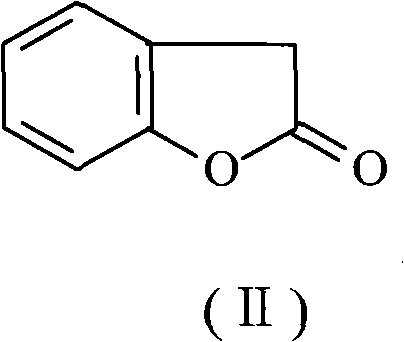
Preparation method of benzofuran-2-(3H)-ketone
A technology of benzofuran and o-hydroxyphenylacetic acid, which is applied in the field of preparation of benzofuran-2--one, can solve the problems of reduced yield, reduced yield, environmental pollution, etc., and achieves a small degree of environmental pollution and avoids raw materials Effect of carbonization and low acidity of wastewater
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
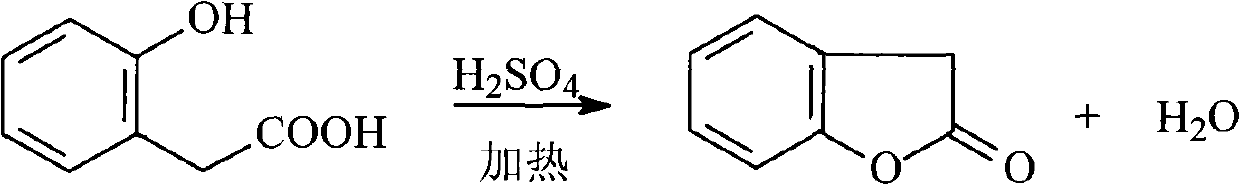
[0036] Add 0.3mol o-hydroxyphenylacetic acid and 3mol toluene into a 500ml three-necked flask, install a water separator, turn on the stirrer and heat the three-necked flask, set the stirring speed to 700r / min, when the temperature of the liquid in the three-necked flask rises to Increase the stirring speed to 850r / min at 60°C, add 0.75ml of dilute sulfuric acid with a concentration of 8mol / L dropwise to the three-necked flask at a speed of 0.15ml / min, and reduce the stirring speed to the speed before the dropwise addition , the temperature of the mixture in the there-necked flask was raised to 135°C for 6 hours, and then the reaction was terminated. After the reaction solution was cooled to room temperature, it was filtered. The filtrate was washed with saturated sodium bicarbonate solution and water successively, and then dried and desolvated under reduced pressure to obtain the target product, the product The purity was 98.3%, and the yield was 99.2%.
Embodiment 2~ Embodiment 9
[0038] The preparation process is the same as in the examples, and the specific process parameters, product purity and yield are listed in Table 1.
[0039] The processing parameter of table 1 embodiment 2-9 and product purity and yield
[0040]
[0041]
[0042] Process parameter and product purity and yield of continued table 1 embodiment 2-9
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com