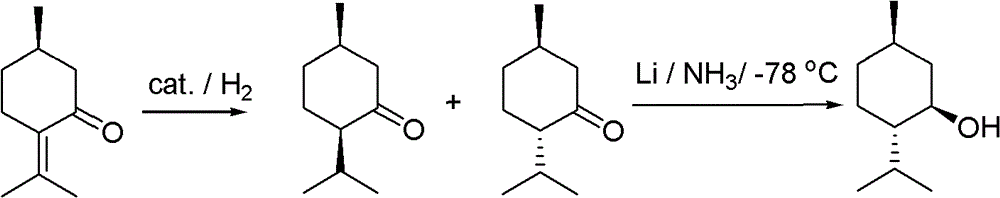
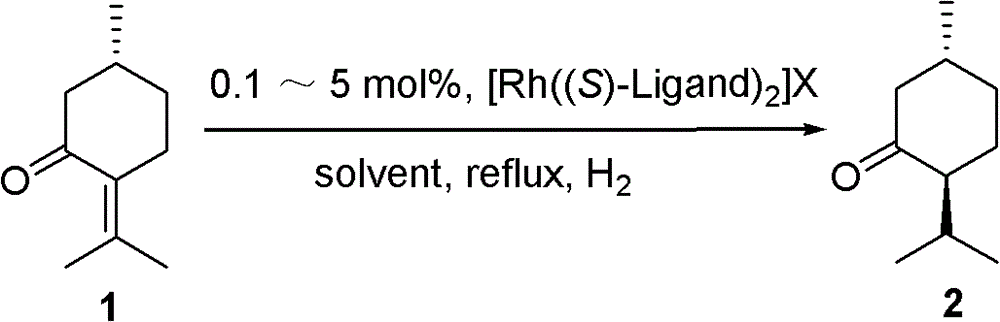
Method for chiral synthesis of levorotatory menthol
A technology for levomenthol and chiral synthesis, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxy compounds, etc., can solve the problems of low total yield, complex and harsh process, poor selectivity and the like, and achieves The effect of simple recovery, good selectivity and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Catalyst Preparation
[0036] (1) Catalyst precursor [Rh(COD)Cl] 2 Synthesis
[0037] Add rhodium trichloride trihydrate (2.0g, 7.6mmol) in the 150mL three-necked flask, after putting into the magnetic stirring bar, add 95% ethanol (30mL), water (12mL) and 1,5- Cyclooctadiene (6mL, 49mmol), stirred and refluxed for 24h, an orange-yellow precipitate was generated, cooled to room temperature, filtered, and the product was washed with cold methanol-water mixture to remove unreacted 1,5-cyclooctadiene, and then used Soak the solid with a little cold ether, then dry it in vacuum at 25°C for 8h, remove the solvent, and obtain orange-yellow crystal [Rh(COD)Cl] 2 (1.593 g, 85% yield) m.p. 243°C.
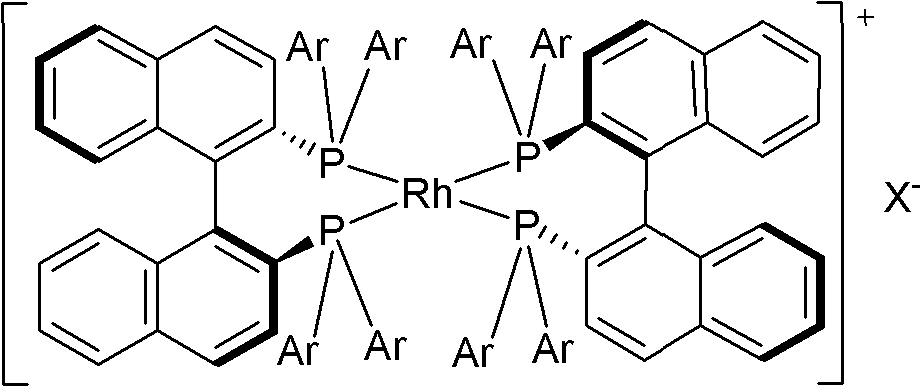
[0038] (2) Catalyst [Rh((S)-p-Tol-BINAP) 2 ] BF 4 preparation of
[0039] Under nitrogen protection, add 0.123g (0.25mmol) [Rh(COD)Cl] in a 25mL round bottom flask 2 and 0.339g (0.5mmol) (S)-p-Tol-BINAP, add 5.0mL freshly distilled CH 2 Cl 2 The solid was dissolved, placed...
Embodiment 2
[0047] 1. Catalyst Preparation
[0048] (1) Catalyst precursor [Rh(COD)Cl] 2 Synthesis
[0049] Add rhodium trichloride trihydrate (1.0g, 3.8mmol) and anhydrous potassium carbonate (0.55g, 4mmol) in a 100mL three-necked flask, add 95% ethanol (17mL), water (8.5mL) under nitrogen protection and 1,5-cyclooctadiene (3mL, 24.5mmol), put it into a magnetic stirrer, stir and reflux for 24h, produce an orange-yellow precipitate, cool to room temperature, filter, and the product is washed with cold methanol-water mixture to remove unreacted 1,5-cyclooctadiene, and finally soak the solid with a little cold ether, then dry it in vacuum at 25°C for 8h, remove the solvent, and obtain the orange-yellow product [Rh(COD)Cl] 2 (0.778 g, 83% yield) m.p. 242°C.
[0050] (2) Catalyst [Rh((S)-BINAP) 2 ] BF 4 preparation of
[0051] Under nitrogen protection, add 0.15g (0.3mmol) [Rh(COD)Cl] to a 10mL round bottom flask 2 , 3 mL of freshly distilled THF and 2.5 mL of 70% HBF 4 , add a mixtu...
Embodiment 3
[0059] 1. Catalyst preparation
[0060] (1) Catalyst precursor [Rh(COD)Cl] 2 Synthesis
[0061] Add rhodium trichloride trihydrate (0.5g, 1.9mmol) in a 50mL three-necked flask, add 95% methanol (9mL), water (4mL) and 1,5-cyclooctadiene (0.14g , 122mmol), put into a magnetic stirrer, stir and reflux for 24h, produce orange-yellow precipitate, cool to room temperature, filter, the product is washed with cold methanol-water mixture to remove unreacted 1,5-cyclooctadiene, and finally use Soak the solid with a little cold ether, then dry it in vacuum at 25°C for 8h, remove the solvent, and recrystallize with dichloromethane / ether to obtain the orange-yellow product [Rh(COD)Cl] 2 (0.393 g, 84% yield) m.p. 243°C.
[0062] (2) Catalyst [Rh((S)-XylBINAP) 2 ] BF 4 preparation of
[0063] Under nitrogen protection, add 0.47g (0.95mmol) [Rh(COD)Cl] to a 10mL round bottom flask 2 Add 0.209g (1.9mmol) NaBF 4 , put into a magnetic stirrer, add 3mL tetrahydrofuran to dissolve and quic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com