Method for producing phenol by oxidizing benzene through nitrous oxide
A technology for nitrous oxide and benzene oxidation, which is applied in the production of bulk chemicals, chemical instruments and methods, and the preparation of organic compounds. It can solve problems such as difficult desorption, carbon deposition on catalysts, and deactivation, so as to avoid overheating The effect of sintering, slowing down the deactivation of carbon deposition and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
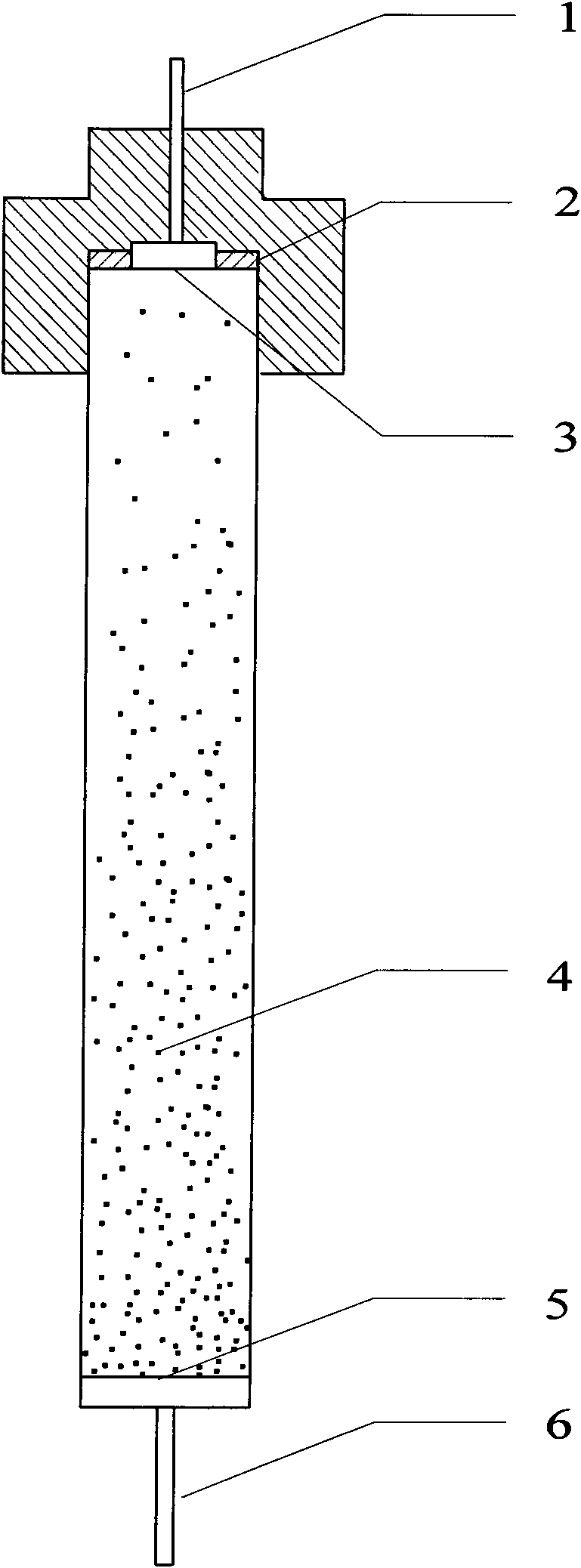
[0021] Example 1: Select 20 grams of H-ZSM-5 molecular sieve with a Si / Al ratio of 25, and put it into a three-necked flask as a carrier. Weigh 1.448 grams of Fe (NO 3 ) 3 9H 2 O, add 300 milliliters of distilled water to prepare a solution, pour this solution into a three-necked flask equipped with 20 grams of H-ZSM-5, reflux and stir at 100°C for 3 hours, then wash with deionized water and filter for 3 times, and then Dry at 110° C. for 12 hours, bake at 540° C. for 4 hours, and after cooling, grind the catalyst tablet into 180-300 μm particles. Then, steam is introduced into the fixed-bed reactor to carry out hydrothermal treatment on the catalyst at 650° C., and the Fe-ZSM-5 molecular sieve catalyst is obtained after cooling.
[0022] Load the prepared catalyst into the gas distribution plate in the fixed fluidized bed reactor of the present invention, activate it with helium at 500°C for 5 hours, and then feed nitrous oxide, benzene and diluent gas under normal pressur...
Embodiment 2
[0023] Embodiment 2: Catalyst preparation process is the same as embodiment 1. The reaction temperature is 500° C., and other reaction conditions remain unchanged. The highest yield of phenol can reach 68.51%, and the yield is still maintained above 61% after continuous reaction for 155 minutes.
Embodiment 3
[0024] Embodiment 3: The catalyst preparation process is the same as in Embodiment 1. Raw material benzene / N 2 O molar ratio is 13, and other reaction conditions are unchanged. The highest yield of phenol can reach 83.18%, and the yield is still maintained above 74% after continuous reaction for 155 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com