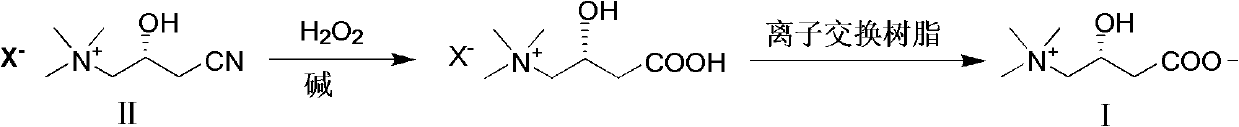
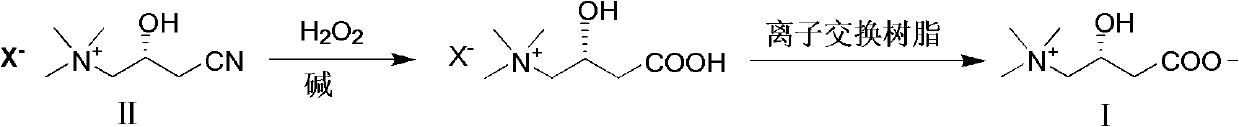
Synthesis method of L-carnitine
A synthesis method and technology of L-carnitine, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of high ammonia nitrogen value in wastewater and high equipment requirements, achieve high reaction yield, short reaction time, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The ratio of the amount of feed material L-(-)-3-cyano-2-hydroxypropyltrimethylammonium chloride:hydrogen peroxide:basic compound is 1:10:2.
[0021] In a 250mL three-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add 17.8g (100mmol) of L-(-)-3-cyano-2-hydroxypropyltrimethylammonium chloride, slowly add 30% peroxide 120g (1000mmol) of hydrogen and 8g (200mmol) of sodium hydroxide, heat up to 70°C and react for 1.5h. After the reaction, cool down, add sodium sulfite until no bubbles are formed, concentrate the reaction solution, adjust the pH to about 5 with acid, pass through Amberlite IRA -400 anion resin exchange column, elute with distilled water, collect the eluent containing product, concentrate, then obtain L-carnitine 12.1g with ethanol-acetone (volume ratio 2: 1) recrystallization, product yield 75.1%, mp 196~197℃, [α] D 20 -32° (c 10, H 2 O).
Embodiment 2
[0023] The ratio of the amount of feed material L-(-)-3-cyano-2-hydroxypropyltrimethylammonium chloride:hydrogen peroxide:basic compound is 1:10:4.
[0024] In a 250mL three-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add 17.8g (100mmol) of L-(-)-3-cyano-2-hydroxypropyltrimethylammonium chloride, slowly add 30% peroxide 120g (1000mmol) of hydrogen and 16g (400mmol) of sodium hydroxide, heat up to 70°C and react for 1.5h. After the reaction, cool down, add sodium sulfite until no bubbles are formed, concentrate the reaction solution, adjust the pH to about 5 with acid, pass through Amberlite IRA -400 anion resin exchange column, elute with distilled water, collect the eluate containing product, concentrate, then obtain L-carnitine 12.8g with ethanol-acetone (volume ratio 2: 1) recrystallization, product yield 79.5%, mp 196~197℃, [α] D 20 -31.7° (c 10, H 2 O).
Embodiment 3
[0026] The ratio of the amount of feed material L-(-)-3-cyano-2-hydroxypropyltrimethylammonium chloride:hydrogen peroxide:basic compound is 1:10:6.
[0027] In a 250mL three-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add 17.8g (100mmol) of L-(-)-3-cyano-2-hydroxypropyltrimethylammonium chloride, and slowly add 40% peroxide Hydrogen 90g (1000mmol) and potassium hydroxide 33.6g (600mmol), heat up to 65°C and react for 1.0h, after the reaction, cool down, add sodium sulfite until no bubbles are formed, concentrate the reaction solution, adjust the pH to about 7 with acid, pass through Amberlite IRA-400 anion resin exchange column, eluting with distilled water, collecting the eluate containing product, concentrating, then recrystallizing with isopropanol-acetone (volume ratio 2: 1) to obtain L-carnitine 13.9g, product yield 86.3 %, mp 196~198℃, [α] D 20 -31.5° (c 10, H 2 O).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com